Information contained in this news release is current as of the date of the press announcement, but may be subject to change without prior notice.
October 10, 2012
Tokyo, October 10, 2012 - Hitachi, Ltd. (TSE:6501, "Hitachi") today announced the development of electrodes which can reduce the cost direct methanol fuel cell (henceforth, "DMFC") batteries by 45% (Hitachi comparison). Specifically, electrodes were developed applying a nitrogen doped carbon catalyst in the air-side electrode (cathode), and a palladium ruthenium alloy catalyst in the fuel-side electrode (anode), eliminating the use of expensive platinum as the catalyst in the electrodes to generate electricity. Development will continue to apply this technology to a compact power source such as portable equipment for outdoor use or disaster situations.
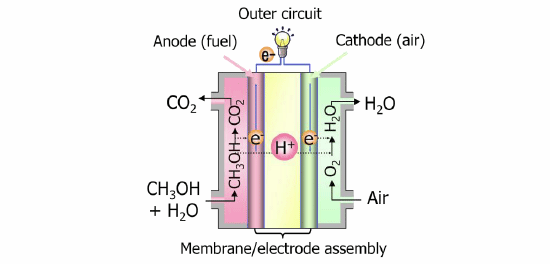
In recent years, there is an increasing need for portable power sources for situations where it is difficult to secure power, such as in the event of a disaster or outdoor situations. Further, from an environmental viewpoint, low carbon dioxide (CO2) emission is also desirable. Liquid fuel cells have attracted attention as a clean power source which can satisfy both requirements. With DMFCs, in particular, as methanol can be directly injected into the fuel cell, an auxiliary unit to manufacture hydrogen is unnecessary, facilitating a smaller size and application as a portable power source.
The electrodes which are important structures of a fuel cell not only accelerate the oxidation reaction of methanol but are also required to efficiently supply reactants such as methanol and oxygen, and remove by-products such as water and CO2. Until now, platinum or platinum alloy catalyst were used in anode due to their high electrode reactivity but carried the issues of accounting for a major portion of the cost of the fuel cell as well as diminishing power conversion efficiency due the carbon monoxide produced as a byproduct of the oxidation reaction adsorbing on the platinum surface of the anode. Hitachi has been conducting material and systems research and development addressing these issues, and has developed non-platinum electrodes applying nitrogen doped carbon catalyst in the cathode, and a palladium ruthenium alloy catalyst in the anode, making it possible to reduce the costs of the conventional DMFC by approximately 45%. Features of the technology developed are:
Hitachi will continue to contribute to the development of clean power systems reducing environmental load, and their practical applications. This technology will be presented at the Pacific Rim Meeting 2012 on Electrochemical and Solid-State Science being held in Hawai'i, USA, from 7th to 12th October.
Hitachi, Ltd. (TSE: 6501), headquartered in Tokyo, Japan, is a leading global electronics company with approximately 320,000 employees worldwide. Fiscal 2011 (ended March 31, 2012) consolidated revenues totaled 9,665 billion yen. Hitachi is focusing more than ever on the Social Innovation Business, which includes information and telecommunication systems, power systems, industrial, transportation and urban development systems, as well as the sophisticated materials and key devices that support them. For more information on Hitachi, please visit the company's website at http://www.hitachi.com.