Creating Disruptive Technologies for the Next Generation
While regenerative medicine has attracted considerable attention as a revolutionary new form of treatment able to cure difficult diseases that have lacked an effective treatment in the past, a number of challenges remain to be overcome before it can enter widespread use in practice. In 2017, Hitachi opened the Hitachi Kobe Laboratory in the Kobe Biomedical Innovation Cluster, Japan’s foremost biomedical cluster, where it is utilizing its proprietary completely closed automated cell culture technology to work toward achieving wider adoption of regenerative medicine through reliable cell production and cost rationalization, two of the key challenges facing the field. This article reports from the front line where open innovation is being used to work with leaders from academia and industry toward the practical application of regenerative medicine and its industrial development for the treatment of conditions such as age-related macular degeneration and Parkinson’s disease using induced pluripotent stem cells.

Hitachi Kobe Laboratory, Center for Exploratory Research, Research & Development Group, Hitachi, Ltd. Current work and research: Research and development of regenerative medicine. Society memberships: The Japanese Society for Regenerative Medicine (JSRM).

Hitachi Kobe Laboratory, Center for Exploratory Research, Research & Development Group, Hitachi, Ltd. Current work and research: Research and development of regenerative medicine. Society memberships: JSRM, the Society of Polymer Science, Japan (SPSJ), and the Japan Society of Applied Physics (JSAP).

Hitachi Kobe Laboratory, Center for Exploratory Research, Research & Development Group, Hitachi, Ltd. Current work and research: Research and development of regenerative medicine.

Biosystems Research Department, Center for Technology Innovation – Healthcare, Research & Development Group, Hitachi, Ltd. Current work and research: Development of bacterial testing technology. Society memberships: JSRM and the Japanese Society for Biomaterials (JSB).

Hitachi Kobe Laboratory, Center for Exploratory Research, Research & Development Group, Hitachi, Ltd. Current work and research: Research and development of regenerative medicine. Society memberships: JSRM.

Hitachi Kobe Laboratory, Center for Exploratory Research, Research & Development Group, Hitachi, Ltd. Current work and research: Research and development of regenerative medicine. Society memberships: JSRM and the Molecular Biology Society of Japan (MBSJ).

Hitachi Kobe Laboratory, Center for Exploratory Research, Research & Development Group, Hitachi, Ltd. Current work and research: Research and development of regenerative medicine. Society memberships: JSRM and MBSJ.
In 2006, Professor Shinya Yamanaka's team at Kyoto University succeeded in producing induced pluripotent stem (iPS) cells(1). Now, more than a decade later, the field of regenerative medicine is entering a new phase of practical application in which iPS cells (a product of Japanese research) provide the basis for a variety of revolutionary new treatments for previously difficult-to-treat diseases. It is against this background that Hitachi has since FY2002 been developing automated cell culture systems that feature a completely closed configuration with the aim of helping regenerative medicine enter widespread use. In 2017, this research and development was relocated from Hatoyama in Saitama Prefecture to the Kobe Biomedical Innovation Cluster (KBIC) in Hyogo Prefecture, where Hitachi is actively engaging in open innovation. Development is also being expedited through collaborative creation with leaders in the field of regenerative medicine. Through these measures, Hitachi is working toward the practical application of automated cell culture systems in the production of cells for medical use.
This article describes what the Hitachi Kobe Laboratory is doing at the KBIC and looks at the future made possible by regenerative medicine.
Driven by work toward the clinical application of cells derived from iPS cells in the treatment of a variety of different diseases, the size of the regenerative medicine market is predicted to grow rapidly from 2020 onwards, reaching 17 trillion yen by 2030(2).
In 2014, Project Leader Masayo Takahashi and her team at the Institute of Physical and Chemical Research (RIKEN) were the first in the world to successfully treat a patient suffering from age-related macular degeneration by transplanting a retinal pigment epithelium (RPE) cell sheet derived from iPS cells harvested from the patient's own epidermal tissue, reporting that the patient was still doing well two years later (this type of transplantation from the patient's own body is called “autotransplantation”)(3). While this result was well received, the time and cost involved in culturing, processing, quality assurance and so forth remain major obstacles to the use of iPS cells to manufacture cells for clinical use. In 2017, clinical research commenced on the transplantation into patients' retinas of a suspension of RPE cells produced from a stock of healthy donor iPS cells held by the Kyoto University Center for iPS Cell Research and Application (CiRA) (transplantation from a donor is called “allotransplantation”). In 2018, with the aim of gaining coverage under public health insurance, the team of Professor Jun Takahashi at CiRA embarked on a clinically led trial in which progenitors of dopaminergic neurons were prepared from a stock of iPS cells and transplanted into the brain of a patient with Parkinson's disease (allotransplantation). Clinical research is also scheduled to commence in the near future on the use of iPS cells in transplantation therapies for patients with serious heart failure and corneal injury at Osaka University and for patients with spinal injuries at Keio University.
Regenerative medicine can involve either autotransplantation of the patient's own cells or allotransplantation of cells from a donor. When using iPS cells for regenerative medicine, autotransplantation invariably has a lower risk of immunological rejection. The problem however, as mentioned above, is the time and cost of preparation, which involves producing iPS cells from the patient's somatic cells and then using these to prepare the required tissue or cells to order. Allotransplantation, in contrast, is likely to be less expensive because it involves producing iPS cells from a donor in large quantities and using these to prepare the appropriate tissue or cells as needed. Human leucocyte antigens (HLAs) are proteins involved in immunological rejection. The CiRA at Kyoto University holds a stock of iPS cells for use in regenerative medicine that were produced from cells harvested by collecting skin and blood from healthy volunteers whose HLA profiles are less likely to result in rejection. The iPS cells were subject to a variety of quality tests and have been made available for medical use and to research institutions and companies since 2015. As of the end of 2018, this stock covered the HLA profiles of approximately 32% of the Japanese population(4). The hope is that, by selecting iPS cells from this stock that match the HLA profile of the patient and using these to prepare the tissue or cells needed for treatment, the level of immunological rejection in allotransplantation can be reduced.
If use of iPS cells is to become a standard medical practice in the future, it will be important to overcome the various specific obstacles to large-scale production so as to enable the supply of high-quality cells manufactured from iPS cells at a reasonable price and to deliver them reliably to the patient. At present, the production of safe therapeutic cells suitable for transplantation relies on their being cultured by an experimental technician in a culturing clean room using manual procedures. Unfortunately, the associated labor costs and the cost of maintaining the culturing facilities are among the reasons why this process is so expensive. Moreover, because cell quality may vary depending on the skill of the technician, how to guarantee that the quality satisfies the standards needed for transplantation is another important question for cell manufacturing. Another risk is that of biological contamination resulting from human involvement in the process.
The Hitachi Kobe Laboratory has been working on the development of automated cell culturing for regenerative medicine as a way of addressing these issues using Hitachi technology.
Hitachi commenced development of automated cell culturing in 2002, work that over the subsequent 15 years culminated in Hitachi's current proprietary technique for completely closed automated cell culturing. Figure 1 shows a diagram of how this completely closed cell culturing works alongside one for the previous method.
Whereas past practice involved working on an open culture inside a CO2 incubator or safety cabinet, the completely closed system is based on a fundamentally different concept. In the completely closed system, the culture module is built with tubes linking the culture plates to the culture fluid bottles or bags, the ends of which are all closed off, and the interior of the module is sterilized by gamma radiation. This is then fitted inside the constant-temperature chamber and culturing commenced by establishing an aseptic connection to fluid with cells in suspension. The 5% CO2 gas required for culturing is supplied intermittently to the culture plates from a cylinder after first being passed through a disk filter (pore size: 0.22 µm) and then via a tube to a humidification bottle. The flow of culture fluid is controlled from outside the tube using a peristaltic pump and involves a fixed cycle of discharge and supply. This configuration provides a high level of safety because culturing can proceed without exposing the interior of the module to the external environment, keeping the risk of contamination by microorganisms or other foreign material very low. As the culturing module is single-use, environmental cleaning after culturing finishes is simple and the risk of cross-contamination is low. Along with these features, the system also ensures reliable quality and enables high-volume production by automating the culturing process that was previously done manually. Figure 2 shows the iACE1 automated cell mass culture system for iPS cells. The system has a completely closed configuration and can be used to culture iPS cells in its ten culture plates. Along with high-volume iPS cell culturing, the system has also been used for the initial differentiation of dopaminergic neuron progenitors. The first system was supplied by Hitachi to the manufacturing plant for regenerative medicine and cell therapy of Sumitomo Dainippon Pharma Co., Ltd., which is engaged in leading-edge work on a global level for the practical realization of regenerative medicine(5). More details are provided below.
Figure 1—Diagrammatic Representation of Open and Completely Closed Cell Culturing Systems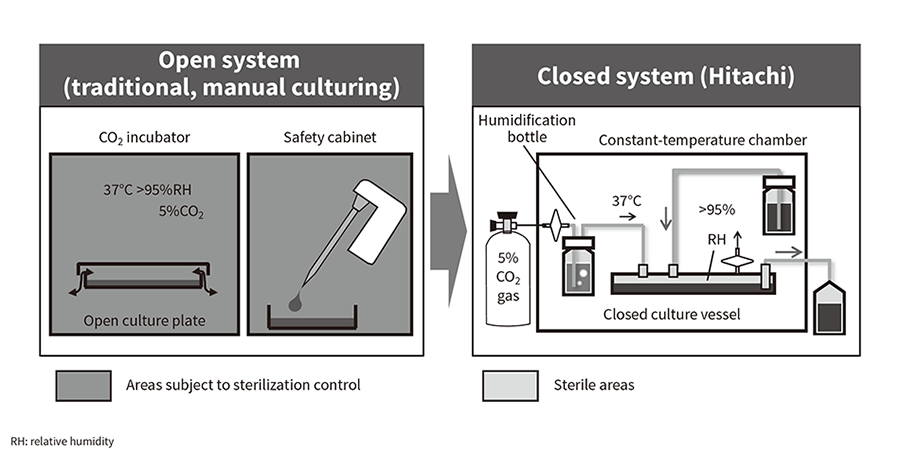 The open culturing practice of the past (left) is subject to contamination risk because of the need to remove the lid of the culture plates in order to work on them. The work also requires sterilization control of a large area. In contrast, the enclosed system (right) has a low risk of external contamination and the sterile area is limited to as small a part of the culturing module as possible.
The open culturing practice of the past (left) is subject to contamination risk because of the need to remove the lid of the culture plates in order to work on them. The work also requires sterilization control of a large area. In contrast, the enclosed system (right) has a low risk of external contamination and the sterile area is limited to as small a part of the culturing module as possible.
Figure 2—Automated Cell Mass Culture System for iPS Cells (iACE1)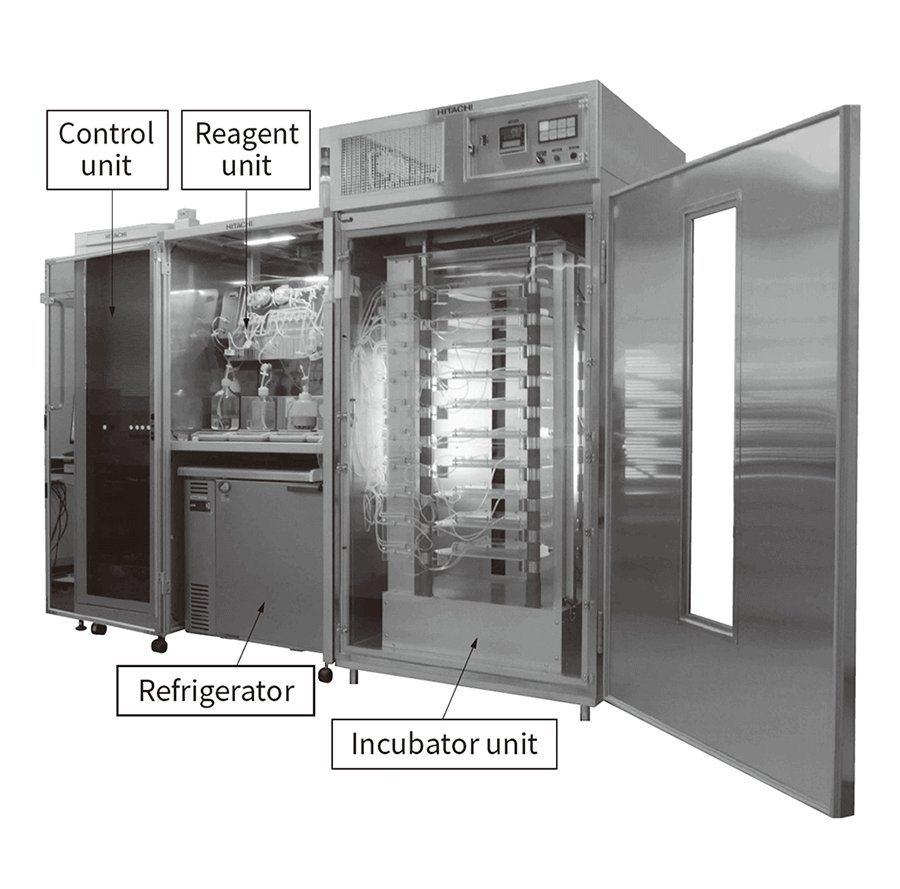 The system is made up of an incubator unit held at a constant 37°C, a reagent unit that supplies the culture medium and other materials, a chiller that holds the culture medium and filtrate during culturing, and a control unit that includes the power supply.
The system is made up of an incubator unit held at a constant 37°C, a reagent unit that supplies the culture medium and other materials, a chiller that holds the culture medium and filtrate during culturing, and a control unit that includes the power supply.
KBIC, the current home of Hitachi Kobe Laboratory, is part of the recovery measures adopted in the aftermath of the Great Hanshin-Awaji Earthquake and was established with the aims of revitalizing the Kobe economy, improving the welfare of local residents, and contributing internationally. The year 2018 marked the 20th anniversary of work first started on the basic concept behind the cluster. The KBIC is made up of a medical cluster, biology cluster, and simulation cluster. Hitachi Kobe Laboratory is located in the Kobe Center for Medical Innovation (KCMI) that was completed in 2017 (see Figure 3). As of March 2019, the site has grown to become Japan's preeminent biomedical cluster, with the involvement of 352 different medical companies or organizations from Japan and overseas, ranging from venture businesses and small or medium-sized enterprises up to major pharmaceutical manufacturers. The awarding in 2018 of a Nobel Prize in Physiology or Medicine to Tasuku Honjo, M.D., Ph.D., President of the Foundation for Biomedical Research and Innovation at Kobe, can be seen as indicative of the nature of the cluster.
Hitachi Kobe Laboratory has been part of the KBIC since 2017(6), engaging in open innovation with leaders in the field of regenerative medicine who are based in the city to pursue the research and development of practical applications for cells derived from iPS cells in which automated cell culturing is a core technology. The following sections provide two examples.
Figure 4—Demonstration of Automated Culturing of RPE Cell Sheets from iPS Cells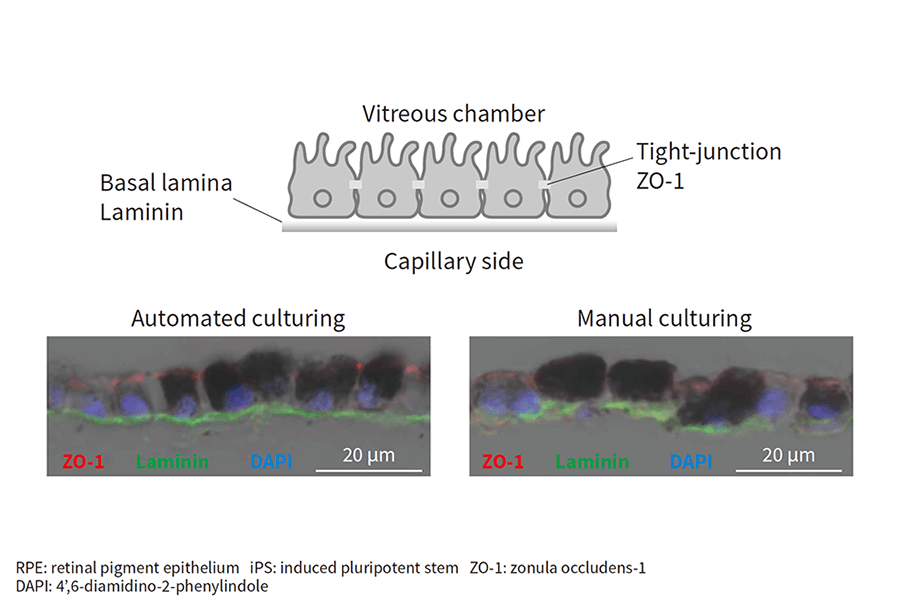 Antibodies specific to a marker of intercellular adhesion (ZO-1, red) and a marker of basal lamina (laminin, green) were detected by fluorometry in vertical cross-sections of cultured sheets of RPE cells. DAPI (blue) indicates cell nuclei.
Antibodies specific to a marker of intercellular adhesion (ZO-1, red) and a marker of basal lamina (laminin, green) were detected by fluorometry in vertical cross-sections of cultured sheets of RPE cells. DAPI (blue) indicates cell nuclei.
The Hitachi Kobe Laboratory commenced joint research with the team of Project Leader Masayo Takahashi at RIKEN in 2016 to trial the completely closed automated cell culturing of RPE cells sheet from iPS cells.
As its name suggests, age-related macular degeneration is associated with aging. A form of eye disease that is difficult to treat, it involves a deterioration in the functioning of the macula at the center of the retina that in serious cases can result in blindness. It is estimated that as many as 690,000 people in Japan potentially suffer from this condition(7). Internationally it is the third largest cause of eyesight loss, imposing large economic costs due to people losing their eyesight as a result of the condition. While a drug treatment is available that slows the progress of degeneration, addressing the root cause of the condition requires transplantation to replace the damaged retinal tissue. While transplants of retinas from aborted fetuses or of tissue from a healthy retina have been taking place since the late 1980s, there is a need for safe and reliable sources of tissue for transplantation as well as ways of overcoming the concerns associated with the treatment, namely immunological rejection of the transplanted tissue, ethical issues, and the highly invasive nature of the procedure(3). As noted earlier, RIKEN has had success with the production of RPE cell sheets from the patient's own iPS cells and their use to treat age-related macular degeneration. Accordingly, Hitachi Kobe Laboratory trialed the automated culturing of RPE cell sheets from iPS cells using automated cell culture equipment 3 (ACE3), a prototype system that had previously been used to automate the culturing of corneal epithelium cells from somatic cells and of sheets of oral mucosal cells. The culture process involved the following steps.
The results are shown in Figure 4. Signals for zonula occludens-1 (ZO-1) (red), a marker of intercellular adhesion, and laminin, a marker indicating formation of a basal lamina (green), were detected for both automated and manual culturing, demonstrating the viability of automated culturing for producing RPE cell sheets from iPS cells(8).
Figure 5—Evaluation of Equivalency between Cells Produced by Automated Culturing (iACE) and Manual Culturing Figure (a) shows the multiplication of iPS cell numbers over a seven-day period for the two methods. Manual culturing used large open culture plates of the same size as those used in the iACE. The sample counts (n) are 10 for iACE and 3 for manual culturing, with the error bars representing one standard deviation either side of the mean. Figure (b) is a comparison of gene expression profiles. A comprehensive assessment was made of the extent of gene expression and a principal component analysis performed. The iPS and intermediate cells were produced by both the iACE and by manual culturing, while the conventional method was used for the production of the end product from the intermediate cells. Even though the production methods use different initial cells, there are no differences in the end product.
Figure (a) shows the multiplication of iPS cell numbers over a seven-day period for the two methods. Manual culturing used large open culture plates of the same size as those used in the iACE. The sample counts (n) are 10 for iACE and 3 for manual culturing, with the error bars representing one standard deviation either side of the mean. Figure (b) is a comparison of gene expression profiles. A comprehensive assessment was made of the extent of gene expression and a principal component analysis performed. The iPS and intermediate cells were produced by both the iACE and by manual culturing, while the conventional method was used for the production of the end product from the intermediate cells. Even though the production methods use different initial cells, there are no differences in the end product.
Through the Evaluation for Industrialization in the Field of Regenerative Medicine project of the Japan Agency for Medical Research and Development (AMED), Hitachi has also been working since 2015 with Sumitomo Dainippon Pharma and Professor Jun Takahashi's team at CiRA at Kyoto University on the development of a process for producing dopaminergic neuron progenitors from iPS cells for use in treating Parkinson's disease.
Parkinson's is a neurodegenerative disease in which the death of dopaminergic neurons results in movement disabilities. It is designated as an intractable disease in Japan. With the number of sufferers in Japan estimated to exceed 160,000, it is also understood to impose high social costs as patients require a high level of nursing care. In addition to drug-based treatment, cell transplantation has also been used since the 1980s to augment the number of dopaminergic neurons and the efficacy of this approach is well-known. Unfortunately, because the cells used for transplantation are obtained from aborted fetuses, the method is problematic in terms of both ethics and cell availability, with side effects also occurring due to the poor purity of the transplanted cells(9). This has led to the idea of instead transplanting dopaminergic neuron progenitors derived from iPS cells, with Professor Jun Takahashi and his team having developed a method for producing dopaminergic neuron progenitors from iPS cells with high levels of efficiency and purity(10). The safety and efficacy of this has already been demonstrated in animal trials(11) and a clinical trial using donor iPS cells commenced in 2018. The product development work for dopaminergic neuron progenitors derived from iPS cells is being undertaken by Sumitomo Dainippon Pharma.
Commercialization requires a way of producing medical-grade cells in large quantities. Accordingly, Hitachi developed the iACE1 automated cell mass culture system for iPS cells described above as a means of utilizing its proprietary technique for completely closed automated cell culturing in the expansion and culturing of iPS cells and in the process for inducing initial differentiation (see Figure 2)(12).
Along with replicating as far as possible the conditions that apply when culturing is done manually, for those conditions that are fundamentally different to those of manual culturing, the work also involved verifying that cells cultured by the automated process were equivalent in quality to those cultured manually with respect to each of the changes made to enable automation. These changes included culturing in a closed environment with fluids and gases supplied via tubes. In this way, practices (protocols) were established for each step in the automated culturing process.
Using these automated culturing protocols, Hitachi succeeded in using its iACE1 for the mass culturing of iPS cells and the subsequent step of inducing initial differentiation. After aseptic harvesting of these cells produced by automated culturing, Sumitomo Dainippon Pharma proceeded with the remaining processes, comparing the quality of the cells against those produced by manual culturing at each step.
The results are shown in Figure 5. Figure 5 (a) shows the multiplication of cell numbers after the expansion and culturing of iPS cells, indicating that this is similar for manual and automated culturing. Figure 5 (b) shows a comparison of the respective profiles obtained by principal component analysis in a comprehensive analysis of gene expression in the iPS cells after expansion and culturing, the intermediate cells after initial differentiation was induced, and in the end product after further differentiation. The points for manual and automated culturing appear in similar parts of the graph in each case, indicating a similar level of quality at the gene expression level also.
The iACE1 not only enables the mass culturing of iPS cells to provide a source of cells for regenerative medicine, when adherent culturing is used, it can also serve as a general-purpose system with applications beyond inducing neuron differentiation. Hitachi intends to continue developing the system to drive the wider adoption of regenerative medicine by using it to produce a wide variety of cell types.
It may be that, a decade from now in 2030, the use of cells and tissue produced from iPS cells will be successfully treating a number of otherwise difficult-to-treat diseases. The volume production and reliable supply of cells for medical use will be essential to the wider adoption of regenerative medicine. Similarly, for use of regenerative medicine to extend beyond the major facilities where it is currently used, and with regard to the transfer of technology for cell culturing, automated culturing has the potential to play a large role in ensuring that cells are produced with consistent quality regardless of location or the technician performing the work. For these reasons, Hitachi intends to continue with the development of technology for automated culturing that can contribute to the wider adoption of regenerative medicine based on the challenges and needs, now and in the near future.
Autotransplantation uses iPS cells produced from the patient's own cells to prepare the cells required for treatment. While the time and cost of producing these iPS cells are an issue, autotransplantation also embodies the ideal of personalized medicine, having major benefits for the patient in the form of a low risk of immunological rejection. In a presentation given in February 2019, Professor Yamanaka expressed his intentions of “demonstrating iPS cells produced from a patient's own cells at Expo 2025 in Osaka, Kansai.” He talked about his hopes of overcoming the obstacles to the production of iPS cells and being able to supply them at low cost (around one million yen). Hitachi is working with the University of Tokyo and Kyoto University on the development of technology for producing a patient's own iPS cells based on a cell manipulation technique that uses microchannels. The intention is to utilize open innovation to speed up the pace of research and development in the future, using specific targets for cost and time as incentives.
This article has described progress toward the practical realization of regenerative medicine using iPS cells and what Hitachi Kobe Laboratory is doing in this field.
While there remain many obstacles to bringing about the future society regenerative medicine will make possible in which people live long and healthy lives, Hitachi intends to continue working toward a society in which people everywhere are able to enjoy the benefits of regenerative medicine and previously intractable diseases can be overcome.
Some of the work described in this article was undertaken at the Advanced Interdisciplinary Center for the Establishment of Regenerative Medicine, part of the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and also “JP18be0104016” of the Japan Agency for Medical Research and Development (AMED). The authors would like to thank everyone involved for their advice and cooperation, including Tokyo Women's Medical University, the Center for iPS Cell Research and Application at Kyoto University, the Institute of Physical and Chemical Research (RIKEN), Sumitomo Dainippon Pharma Co., Ltd., the Foundation for Biomedical Research and Innovation at Kobe, and Kobe City.