Smart Life Solutions for People Everywhere
Regenerative medicine using processed cells is recognized for its potential to treat diseases that have previously been difficult to treat, with research underway globally on getting it to the stage of clinical use. There has also been considerable work done on its commercialization in Japan with a new law on regenerative medicine (Act on Safety of Regenerative Medicine) enforced in November 2014 along with changes to the Pharmaceutical and Medical Device Act. The size of the market for treatments based on regenerative medicine is predicted to reach one trillion yen by 2030 and there is also a strong public interest case for the enhancements it could bring to patients’ quality of life(1). Against this background, Hitachi Global Life Solutions, Inc. is utilizing technology for precisely controlling air quality in terms of temperature, humidity, cleanliness, and pressure to supply the regenerative medicine industry with air conditioning solutions for cell processing facilities. In the process of supplying these solutions, the company draws on its total engineering capabilities for coordinating air conditioning solutions and equipment at cell processing facilities in collaboration with the wide range of other companies involved in facility operation, thereby delivering economic value to customers in forms that include reduced business losses and the provision of the right environment by supplying the air conditioning solutions that they actually want.



Past pharmaceuticals have included the use of low-molecular-weight compounds and cell secretions as medicines. Recent years, however, have seen extensive research into regenerative medicine that involves processing the very cells that make up living organisms in ways that address particular illnesses and using those cells to treat the illness, and there is strong demand for its clinical use.
Currently, regenerative medicine and related products authorized for use in Japan mainly involve the use of stem cells and other cells that are then cultured into the desired cell type or tissue for use in treatment. Induced pluripotent stem (iPS) and embryonic stem (ES) cells can resolve the problem of how to source the stem cells required by regenerative medicine and high hopes are being placed on clinical research in this area. The use of chimeric antigen receptor T cells (CAR T cells) has attracted attention, having been approved for cancer treatment in Japan. This involves extracting the patient’s own T cells and then genetically modifying them for use in treatment.
The cells used in regenerative medicine are mainly produced at what are known as cell processing facilities. These facilities are made up of sterile clean rooms with separate zones for the different processes used, and maintain high levels of sterility in accordance with the stipulated criteria for each zone. A feature of these facilities is that the air cleanliness, temperature, humidity, and pressure differences between rooms are classified into separate grades (see Table 1).
The Air Conditioning Systems Engineering Business Unit of Hitachi Global Life Solutions, Inc. (Hitachi GLS) has put a lot of effort into special-purpose air conditioning applications that utilize control technology for precisely controlling air quality, including clean rooms for medical research and manufacturing, biohazard rooms for testing pathogens, and industrial clean rooms for semiconductor manufacturing. Meanwhile, there has been an upsurge in involvement by private-sector companies resulting from the introduction in November 2014 of the Act on Safety of Regenerative Medicine that permitted the construction of privately-run cell processing facilities. It is against this background that Hitachi GLS is seeking to create a sustainable society by means of air conditioning solutions for the regenerative medicine industry that utilize control technology from special-purpose air conditioning applications.
Table 1—Maximum Permitted Particle Counts in Sterile Clean Room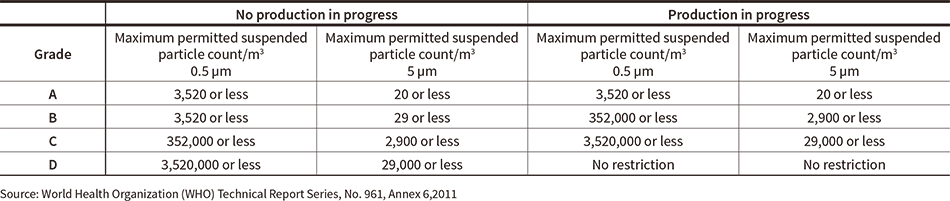 The table lists the different grades of air cleanliness for areas used in sterile pharmaceutical production.
The table lists the different grades of air cleanliness for areas used in sterile pharmaceutical production.
To strengthen its air conditioning solutions business for cell processing facilities, Hitachi GLS identified the following additional four points as important for managing production facilities.
Based on a belief that a more vibrant market for regenerative medicine in Japan would come about if solutions that address these points could be provided, plans were made to build a cell processing facility at Nihonbashi in the Chuo district of Tokyo. As Hitachi GLS handled the production and sale of all of the construction materials and equipment used to build the facility, it set out to overcome the challenges of achieving these four points through collaborative creation with other companies.
In cooperation with Hitachi, Ltd.’s Building Systems Business Unit, Hitachi GLS engaged in collaborative creation on the construction of the cell processing facility with the Life Science bases of Mitsui Fudosan Co., Ltd. in Nihonbashi, Tokyo.
Nihonbashi is home to a number of organizations that work in the life sciences, including pharmaceutical companies and the offices of the Japanese Society for Regenerative Medicine, and Hitachi GLS plans to locate its cell processing facility showroom in the neighborhood. Normal practice at cell processing facilities is to restrict access to only those people needed for the cell processing work and this obviously presents difficulties when wanting to show around customers looking to build such a facility of their own. The Regenerative Medicine Innovation Center, which integrates the latest technologies, was established as a way to overcome this problem, taking advantage of the Nihonbashi location.
The equipment at the center is arranged differently than most other facilities. This is so that collaborative creation partners can bring along the latest equipment equipped with functions that integrate with those of other vendors.
The collaborative creation partners share a common desire to boost activity in the market for regenerative medicine, and the aim is for engineering work that looks ahead to the future automation of equipment and systems to be undertaken in parallel with “One HITACHI” initiatives.
The following subsections (1) through (5) describe features of the cell processing facility at Hitachi GLS’s Regenerative Medicine Innovation Center.
Hitachi GLS is also working to coordinate equipment for future automation requirements, such as the installation of incubators equipped with an automatic culture replacement unit.
Keeping equipment functional through ongoing maintenance and regular validation are important tasks at a cell processing facility. Hitachi GLS has its own vendor service network that provides the infrastructure for customers to use their air conditioning systems with confidence.
Given that depopulation and other issues associated with aging demographics and a low birthrate will make the recruitment and skills transfer of maintenance staff more difficult for customers in the future, technology that uses the IoT to deal pre-emptively with problems in air conditioners will be essential. It is also anticipated that manufacturing will shift away from manual operation and toward greater automation in order to ensure more reliable quality.
In the future, Hitachi GLS intends to proceed with the development of concepts for the next generation of cell processing facilities that will feature modularization and greater efficiencies, working with customers and collaborative creation partners to enhance the automation needed for these regenerative medicine facilities and the use of workflow planning to increase productivity per unit of floorspace. The Regenerative Medicine Innovation Center will play a central role in this work. With regard to product development for the equipment used in clinics, as many cell processing facilities will be located in leased buildings, the company will also develop proposals that include automation at the Regenerative Medicine Innovation Center, which is itself located in a leased building in Nihonbashi.
This article has focused on the Regenerative Medicine Innovation Center to describe the work being done by Hitachi GLS on an air conditioning solution for the regenerative medicine market, one of the company’s special-purpose air conditioning solutions. The technology of regenerative medicine is advancing rapidly and Hitachi GLS intends to keep pace with this progress, continuing to publish information so that it can continue supplying solutions that contribute to society as well as business growth.
Considerable advice and assistance were received from the various parties concerned in the construction of the Regenerative Medicine Innovation Center described in this article, including Mitsui Fudosan Co., Ltd., Nikkei Panel System Co., Ltd., RORZE Lifescience, Inc., Santasalo & Steri-Pro Solution Corporation, Nikon Corporation, and Cyfuse Biomedical K.K. The authors would like to take this opportunity to express their deepest gratitude.