1. Big Data Analysis Platform for the Medical Field
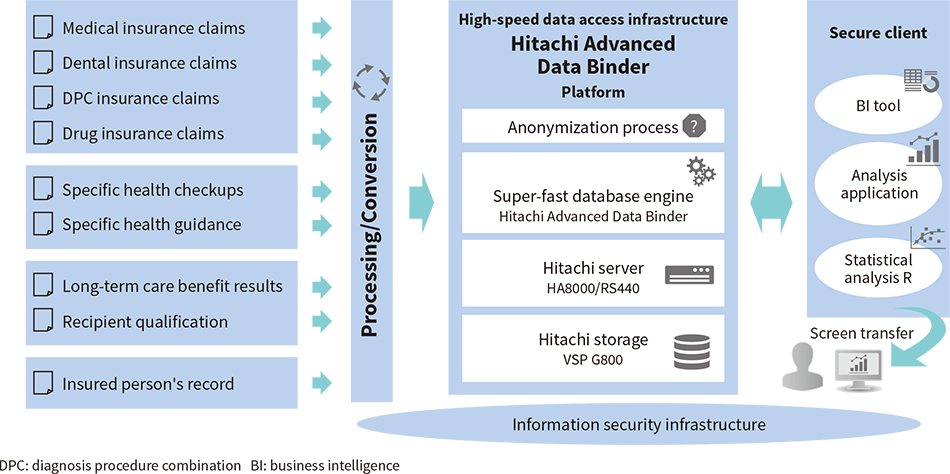
The Japan Agency for Medical Research and Development (AMED) is a Japanese organization that promotes medical research and development. The Institute for Health Economics and Policy (IHEP) acts as the representative institution of an AMED-sponsored program on ICT Infrastructure Development for Clinical Research called “Research on Super-fast Super-interdisciplinary Next-generation NDB*1 Data Research Infrastructure Development to Enable the Rapid Creation of Evidence.” Hitachi was in charge of developing the initial version of the Super-fast Super-interdisciplinary Japanese Medical Insurance Claim Bigdata Analytics Platform System (SFINCS) based on a basic design by the University of Tokyo, which is affiliated with the IHEP.
The SFINCS uses Hitachi Advanced Data Binder*2, a super-fast database engine developed by Hitachi based on the out-of-order execution principle*3, which was developed at the University of Tokyo. Hitachi Advanced Data Binder acts as the database infrastructure for enabling more effective utilization of the NDB stored by the Ministry of Health, Labour and Welfare.
- *1
- NDB: National Database of Health Insurance Claims and Specific Health Checkups of Japan
- *2
- Using the results from the study “Development of the Fastest Database Engine for the Era of Very Large Database and Experiment and Evaluation of Strategic Social Services Enabled by the Database Engine Project” (Principal researcher: Masaru Kitsuregawa), which was supported by the Japanese Cabinet Office's Funding Program for World-Leading Innovative R&D on Science and Technology.
- *3
- Principle devised by Masaru Kitsuregawa (Professor at the Institute of Industrial Science, The University of Tokyo, and Director General of the National Institute of Informatics) and Kazuo Goda (Project Associate Professor at the Institute of Industrial Science, The University of Tokyo).