GLOBAL INNOVATION REPORT
Fusion of Electron Microscopy and Image Analysis in Biological Drug Manufacturing Processes
Hitachi High-Technologies and Vironova AB, Stockholm, Sweden, have initiated a research collaboration to contribute to the efficient development of new biological drugs. There is exciting potential in combining Hitachi High-Technologies’ instruments with Vironova’s automation solutions to help meet growing healthcare needs.







Hitachi High-Technologies and Vironova AB, Stockholm, Sweden, have initiated a research collaboration to explore opportunities where the two companies' core competencies can complement each other to contribute to the efficient development of new biological drugs (biologics) in the pharmaceutical and life science markets.
Hitachi High-Technologies holds a strong position in transmission electron microscopy (TEM) and, with an increasing need for new analytical methods in the pharmaceutical industry, new opportunities are arising.
Vironova is an established provider of electron microscopy to the pharmaceutical industry and has developed proprietary software for TEM system control. Vironova's specialty is the automation of microscope operation together with innovative image analysis using, for example, artificial intelligence (AI) and machine learning.
In the highly-regulated development of biologics, objective, automated image analysis is vital for the future of electron microscopy. Accuracy of results and deep, meaningful data will ultimately speed up development time and save costs.
There is exciting potential in combining Hitachi High-Technologies' instrument expertise and Vironova's automation solutions to help solve growing healthcare needs.
One of the greatest challenges currently facing humankind is the need to combat health problems associated with an increasing and aging population (cancer, dementia, and the spread of infections due to overcrowding and travel) and the rising prevalence of multi-antibiotic-resistant bacterial strains.
At the same time, pharmaceutical development is undergoing a revolution with the use of biological substances as therapeutics.
Biological drugs employ a wide range of substances of biological origin. They include gene therapies, vaccines, recombinant antibodies, and biological molecules such as nucleic acids and so on. They also have in common the fact that the drugs themselves are large molecules, far larger than the small chemicals traditionally used. They can thus be examined using an electron microscope, allowing for direct and detailed analysis of many characteristics.
Biological products often represent the cutting-edge of biomedical research and may eventually offer the most effective means to treat medical conditions with no alternative treatment. They are slowly moving into the mainstream of drug development and one particular class of biologics, gene therapies, is currently receiving much attention for its advances and successes.
Vironova specializes in gene therapy applications and this area is of particular interest in the collaboration with Hitachi High-Technologies. An initial collaborative project has demonstrated a viable technical solution that could be attractive to the pharmaceutical industry and help solve analytical bottlenecks in the development of biological drugs.
After decades of research, the first gene therapy products were recently approved by the U.S. Food and Drug Administration (FDA)(1). The FDA is regulating these products as biologics, giving them twelve-year non-patent exclusivity.
The first two treat certain forms of cancer: Novartis' Kymriah*1 (tisagenlecleucel), for children and young adults with a form of acute lymphoblastic leukemia (ALL), was approved in August 2017, and Gilead Sciences' Yescarta*2 (axicabtagene ciloleucel), for a form of lymphoma, was approved in October 2017. These two therapies are examples of adoptive immunotherapy with chimeric antigen receptor T cells (CAR-T).
CAR T-cell therapies are combined gene therapies and immunotherapies. CAR-T takes immune cells, called T cells, from a patient and genetically engineers them to express chimeric antigen receptors (CARs) that recognize the patient's cancer cells. Cells are then introduced back into the patient's bloodstream where they target and kill the cancer cells. With many early successes in clinical trials using CAR-T, there is great hope it can be used to treat a wide variety of blood and solid tumor cancers. The American Society of Clinical Oncology's (ASCO) Clinical Cancer Advances 2018 report recently named CAR-T as the most important clinical cancer advance of the year.
Cancer is by far the largest group of diseases (65%) being investigated in gene therapy clinical trials. The second largest group (11.1%) is inherited monogenetic diseases. Gene therapy is unique in this area, as it has the potential to correct inherited gene disorders by delivering the missing or damaged gene(2).
In late 2017, Spark's Luxturna*3 was approved to treat children and adults with an inherited disease of the eye. A one-time gene therapy treatment in each eye restores the visual cycle by delivering the corrected gene.
To date, approximately 2,600 clinical trials and 16 gene therapy products have been approved in various countries(2), (3).
The recent clinical success and subsequent increased investment from the market have created an upsurge in innovative companies looking toward manufacturing and commercialization of their gene therapy products.
Gene therapies represent a new medical paradigm, but even before they reach the patient and medical profession they give rise to a large number of issues in their development and manufacturing.
The need to develop suitable analytical tools for gene therapy products has become increasingly evident. Because of their intended use in patients, they must meet rigorous safety guidelines.
The FDA introduced the Process Analytical Technology (PAT) initiative in 2004 for biopharmaceutical manufacturing to emphasize the importance of in-depth analysis throughout the whole production process instead of the more limited traditional approach of process validation and then extensive end product testing. Pharmaceutical companies have begun to investigate new analytical technologies to measure critical quality attributes and allow for better understanding and control during the manufacturing process(2).
Gene therapy introduces genetic material into cells to compensate for abnormal genes or to produce a beneficial protein. In some forms of gene therapy, a carrier, or vector, is genetically engineered to deliver the gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell, the viruses being modified so they cannot cause disease.
The vector used to introduce the gene of interest is a critical aspect of any gene therapy. Most commonly, viral vectors, derived from lentivirus (LV), adenovirus (AV), or adeno-associated virus (AVV) are used. One of the challenges for the characterization and quality control of viral vectors is their degree of complexity. Even recombinant adenovirus associated virus (rAAV), the least complex recombinant viral vector, has a structure more complex than the most complex recombinant proteins.
Key to the development of large-scale, optimized production for viral vectors is access to accurate and reproducible analytical tools to monitor quality attributes that ensure a safe, high-quality, consistent, and efficacious product.
The critical quality attributes in monitoring viral vector manufacturing for gene therapy include viral potency, identity, quantity, purity, aggregation, empty viruses, protein content, and product safety. There are currently various characterization assays available and their relevance depends on the virus as well as the expression system.
In general, biological substances and structures are difficult to handle because they are complex and sensitive mixtures that are not easily purified, characterized, or analyzed. They tend to aggregate, break down, and become contaminated.
Furthermore, the path to a pharmaceutical product is a complex one, from discovery, through development of robust processes, to quality control in routine manufacturing. There are many parameters to track and control so as to avoid undesired issues that could negatively impact the pharmaceutical product. For example, characteristics such as purity (see Figure 1 and Figure 2) and integrity (see Figure 3) can often be directly correlated to the efficacy of the final pharmaceutical product and these are areas in which electron microscopy can contribute significantly.
Figure 1— Sample TEM Images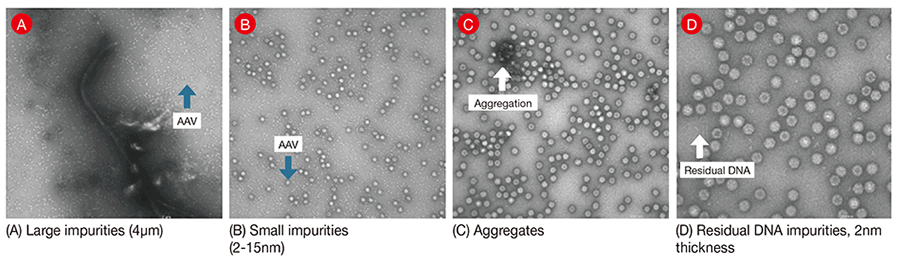 The figure shows examples of the structural expression in transmission electron microscope (TEM) images of contaminants that may appear in, for example, adeno-associated virus samples.
The figure shows examples of the structural expression in transmission electron microscope (TEM) images of contaminants that may appear in, for example, adeno-associated virus samples.
Figure 2— Automated Purity Analysis of Two Samples Using Morphology Classification to Differentiate between Primary Particle and Debris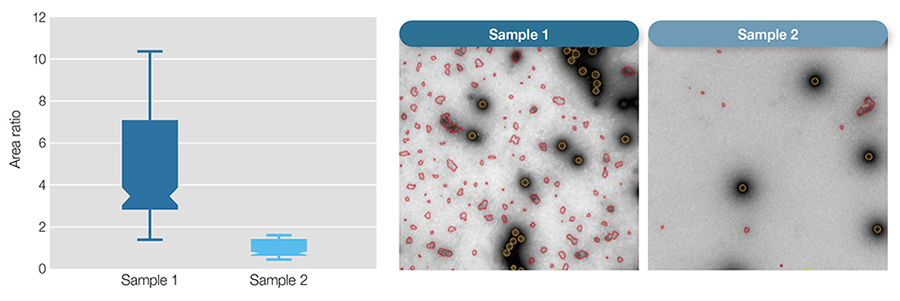 The box-plot shows purity measurements for the two samples calculated as the ratio of the total area of debris to that of adenovirus particles.
The box-plot shows purity measurements for the two samples calculated as the ratio of the total area of debris to that of adenovirus particles.
Figure 3— nsTEM for Revealing Particles with Disrupted Capsid Structure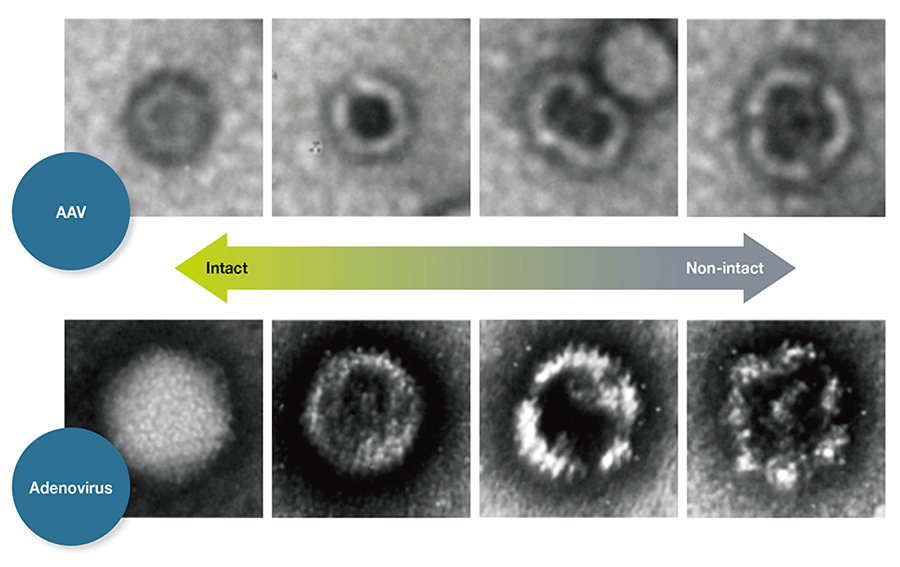 TEM analysis of negatively stained AAV samples (nsTEM) is a reproducible method for revealing particles with disrupted capsid structure. Disrupted particles take in stain and can therefore be identified by their darker centers.
TEM analysis of negatively stained AAV samples (nsTEM) is a reproducible method for revealing particles with disrupted capsid structure. Disrupted particles take in stain and can therefore be identified by their darker centers.
Electron microscopy imaging is one of the most powerful tools for investigating complex biological systems. It provides a combination of visual proof and metric values that is not achievable from the indirect methods currently used for monitoring pharmaceutical processes.
However, it is essential to automate processes and analyses in order to render electron microscopy suitable for use in the pharmaceutical industry. Automation provides robustness, lower variability, higher reproducibility, and higher throughput. Faster access to informative analytical data leads to better informed decisions and earlier optimization of processes.
Vironova's specialty is the automation of microscope operation together with innovative image analysis using, for example, AI and machine learning. This may be seen to be part of a larger movement in the pharmaceutical industry to improve the efficiency of manufacturing via the implementation of digital technologies, sometimes known as “Industry 4.0(4).”
In the first feasibility study carried out using the Hitachi 120kV TEM system HT7800 and Vironova automation software, analysis of viruses in gene therapy was exemplified by studying a new type of gene therapy vector called phage therapy.
The practice of phage therapy, which uses bacterial viruses (bacteriophages, or phages) to treat bacterial infections, has been around for almost a century but the universal decline in the effectiveness of antibiotics has renewed interest in this practice(5).
The results of the study provided a useful solution that gave detailed data on sample quality and significantly reduced the learning time for performing the study, thanks to the automation of several key microscope operating steps, such as beam alignment that only required 10 % of the usual time.
Additionally, the sample analysis itself was significantly less operator-intensive thanks to automated screening of the sample grid (see Figure 4 and Figure 5). Arguably the most powerful aspect of this solution is the attainment of quantitative data due to the image analysis in which critical quality attributes of the bacteriophages could be confirmed and deviations quantified.
Gene therapy, along with biologics in general, is a young field where the establishment of production protocols is still in its infancy. Understanding of the parameters affecting gene therapy patient safety is still evolving and the needs of the field are diverse. As more projects move into clinical phases, and as manufacturers develop scaled-up commercial processes, there are further challenges.
Optimizing process steps to ensure final product quality and patient safety can be a daunting task. Having access to a combination of data from reliable analytical solutions is key to success. TEM provides images of the actual content of the sample, and subsequent automated image analysis converts the images into accurate metrics that reflect sample quality.
The particular challenges of gene therapy development represent an opportunity to combine the existing Hitachi High-Technologies electron microscopy and Vironova automated image analysis platforms into a new offering that can lead the field from the start and contribute significantly to developing the potential of this exciting new area.
This is a good example of integrating cyber technology with the physical world of electron microscopy for the ultimate purpose of helping to solve growing healthcare needs. We all have a vested interested in ensuring the safest product possible can be manufactured for these new therapies.