IP Activities for Supporting Corporate Management in the Digital Era
Particle beams are recognized as having considerable potential for treating cancer in ways that minimize the stress on the patient, and are being deployed for this purpose both in Japan and overseas. Hitachi has also been working on the development of a particle beam therapy system through collaborative creation with a university. Specifically, one of the outcomes of the Advanced Radiation Therapy Project: Real-time Tumor-tracking with Molecular Imaging Technique through Japan’s Funding Program for World-Leading Innovative R&D on Science and Technology, on which Hitachi worked jointly with Hokkaido University, was the commercialization in 2014 of a scanning irradiation technique that can track moving organs and that combines the spot scanning technique developed by Hitachi with real-time tumor-tracking radiation therapy developed by Hokkaido University. Subsequently, the invention jointly developed by this work was chosen for the Imperial Invention Prize, the top prize at FY2017 National Commendation for Invention awards. Development of the technology is ongoing through a process of collaborative creation with the intention to contribute to the wider adoption of particle beam therapy.



The use of radiation to treat cancer has spread in recent years because of its ability to provide a high quality of life (QoL) for patients after treatment. This has included growing interest in particle beam therapy (which uses beams of protons or carbon ions that have been accelerated to a high energy) for its potential to deliver a highly concentrated dose of radiation to the tumor, thereby improving the efficacy of therapy and reducing side effects. Hitachi develops its particle therapy systems by drawing on expertise built up through the development and manufacture of particle accelerators for use in physics research(1). A proton beam therapy system delivered to the University of Tsukuba Hospital Proton Radiation Research Center, the first such system intended as a dedicated therapeutic facility, commenced operation in 2001(2). Hitachi received an order in 2002 for a proton beam therapy system from the University of Texas MD Anderson Cancer Center (MDACC), one of the world's leading cancer clinics, which became the first commercial facility in the world to commence therapy using proton beam spot scanning in 2008(3), (4).
Spot scanning is able to further enhance the ability of particle therapy to precisely target the dose. A problem, however, is that full use cannot be made of this precise targeting if the tumor is moving for reasons such as the patient's breathing. To overcome this, Hitachi teamed up with Hokkaido University to develop technology that combines the Hitachi-developed spot scanning with a university-developed tumor tracking technique that indicates the position of a moving tumor(5). This joint development was also selected in 2009 for inclusion in the Advanced Radiation Therapy Project: Real-time Tumor-tracking with Molecular Imaging Technique through Japan's Funding Program for World-Leading Innovative R&D on Science and Technology. Although the project encountered a number of technical challenges along the way, they worked together to overcome these difficulties and successfully completed system development. The completed system, the PROBEAT-RT, was supplied to Hokkaido University and, after being approved for manufacture and sale as a medical device under the Pharmaceutical Affairs Act in February 2014, proton beam therapy based on spot scanning commenced in March of that year. Approval for manufacture and sale as a medical device under the Pharmaceutical Affairs Act was also obtained for a therapy system that combines tumor-tracking and spot scanning in August 2014, with therapy commencing from December of that year. The technology has been deployed to date at eight facilities in Japan and elsewhere where it is current being prepared to commence therapeutic use so as to be available to treat a larger number of patients. This article describes the joint development of techniques for targeting the particle beam with high accuracy at tumors that are moving due to respiration or similar.
Hitachi's proton beam therapy system uses a synchrotron, a form of circular accelerator, to accelerate protons to about 70% of the speed of light. The proton beam is extracted from the synchrotron, passes along the high-energy beam transport line to a rotating gantry that can aim the proton beam at the patient from any direction, which is done via an irradiation nozzle that shapes the proton beam to match the dose distribution to the shape of the patient's tumor. Figure 1 shows the configurations of the beam-shaping mechanisms for spot scanning and for the previous method that used scattering irradiation. In the case of scattering irradiation, the proton beam enters the irradiation field shaping mechanism with uniform energy. Here, the high dose rate region is enlarged using devices that spread the beam in the depth and lateral directions respectively. These devices utilize the principle that a proton beam is spread by scattering as it passes thorough matter, losing energy as it does so. After spreading, a beam-limiting collimator is used to match the beam cross-section to that of the tumor. In contrast, the spot scanning technique developed by Hitachi seeks to direct a narrow proton beam from the accelerator at the target without allowing its diameter to spread as much as possible, and matches the dose distribution to the tumor shape by three-dimensional scanning of the irradiation site. The irradiation depth (in the direction of the proton beam) is adjusted by varying the energy to which protons are accelerated by the synchrotron, and two scanning magnets are used to electromagnetically scan the beam over the plane perpendicular to the beam direction. The greater control this provides over the resulting dose distribution allows it to be matched to the shape of the tumor.
Figure 1—Comparison of Beam-shaping Mechanisms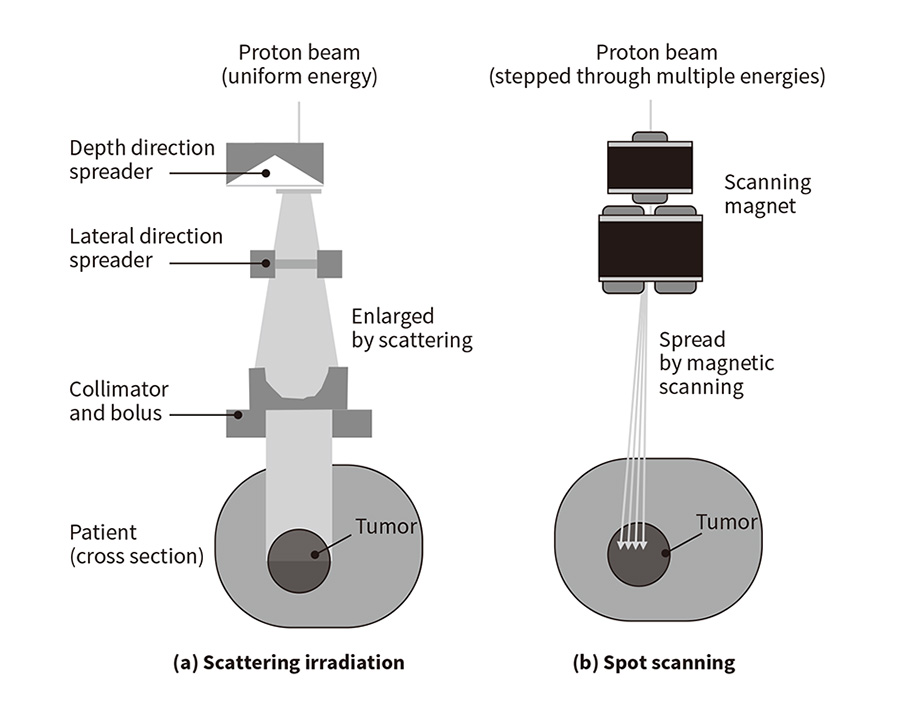 (a) Scattering irradiation uses scattering to spread the beam and then a collimator to shape it to match the shape of the tumor.
(a) Scattering irradiation uses scattering to spread the beam and then a collimator to shape it to match the shape of the tumor.
(b) Spot scanning uses scanning and changes in energy level to progressively scan the beam over the tumor, thereby achieving a dose distribution that matches the shape of the tumor.
To provide proton beam therapy for organs such as the lungs or liver that move due to respiration and other bodily functions, Hitachi has worked with the University of Tsukuba Hospital Proton Medical Research Center to offer an irradiation technique called respiration synchronization. This uses a synchronization device that detects the surface movement of the patient's body to generate a respiration phase signal, with the therapy system only exposing the patient to the proton beam on receiving a gate signal indicating that the movement is at a predetermined phase in the respiratory cycle.
Using the synchrotron operating practices from this respiration-synchronized irradiation method as a base, the newly developed technique combines Hitachi's spot scanning with the real-time tumor-tracking technique(6) developed by Hokkaido University to enable treatment of moving tumors. Figure 2 shows a photograph of the therapy room. Tumor tracking works by injecting a gold marker (1 to 2 mm in diameter) into the patient close to the tumor and obtaining X-ray images from two different directions at a rate of 30 frames per second to determine the location of the gold marker in the patient's body in three dimensions. The beam is then only output when the location of the marker is within a predetermined range. The combination of tumor tracking and spot scanning irradiation enables the movement of the tumor to be determined and ensures precise irradiation of moving organs. The joint research was initially organized on the basis of the Hitachi team taking primary responsibility for research and development while the research team at Hokkaido University handled testing. As the joint development started out with a clear goal of targeting the particle beam based on the position of a gold marker that moved in synch with the tumor, it was assumed that it would be able to proceed without any problems.
Figure 2—Therapy Room at the Proton Beam Therapy Center of Hokkaido University Hospital The room is equipped with the proton beam irradiation system used for therapy and systems for obtaining X-ray images from two different directions to measure the tumor position. The proton beam irradiation system is designed to rotate so as to direct the proton beam at the tumor from any direction.
The room is equipped with the proton beam irradiation system used for therapy and systems for obtaining X-ray images from two different directions to measure the tumor position. The proton beam irradiation system is designed to rotate so as to direct the proton beam at the tumor from any direction.
The joint work with the research team at Hokkaido University started out by addressing the irradiation efficiency and dose distribution when using the combined technique. In this context, irradiation efficiency means the probability that the proton beam will be available when the signal from the tumor-tracking system requests beam output. To improve mutual understanding of a system that would combine spot scanning and tumor tracking, the discussions involved Hitachi providing a detailed explanation of the control techniques used on the synchrotron and Hokkaido University explaining the features of the gate signal output from the tumor tracking system. Unfortunately, it became clear that synchronizing the irradiation timing with tumor movement would be more difficult than anticipated. The operating practice that Hitachi adopted for the previous respiration-synchronized irradiation technique involved switching operation to a standby mode after the protons had been injected into the synchrotron and accelerated, and only extracting the beam when the gate signal turned on. It was initially intended that this same operating practice could be adopted for the system that incorporated tumor tracking. Unfortunately, while tumor tracking determines the gold marker position 30 times a second, the complex and erratic nature of tumor movement means that the gate signal is likely to turn on and off over short time periods. As shown in Figure 3(a), what happens when using the previous operating practice is that the proton beam is only output when the gate signal first turns on, after which the synchrotron accelerator switches to deceleration. When the research team at Hokkaido University made calculations using gate signal data from their previous tumor-tracking radiation therapy technique, which used X rays, they came up with an estimated irradiation efficiency of only 4%. This meant that use of the previous operating practice would result in therapy taking more than one hour, diminishing the viability of the system for clinical use.
Although the Hitachi and Hokkaido University teams engaged in numerous discussions about how to resolve this problem, with a series of visits and videoconferences, they kept ending up back where they started. They looked at overcoming the problem by generating the gate signal based on a prediction of tumor movement. Unfortunately, the Hokkaido University research team knew from experience that methods based on predicting tumor movement were out of the question because of the complexity of human bodily movement. There were also many ideas that had to be ruled out on the basis of development time and cost, such as improvements to the accelerator itself to enable continuous output. Then, just as it felt like they were running out of ideas, they realized that the spot scanning technique previously developed by Hitachi had a function to temporarily halt particle beam output while repositioning the beam. Using this idea as a base, they came up with the scheme shown in Figure 3(b), one that would minimize the amount of changes needed to the existing system. By introducing a new type of standby control after the commencement of irradiation, they added a function to temporarily halt beam output whenever the tumor went outside the irradiation range. This involved an operating practice whereby the function for temporarily halting beam output was given a maximum time length setting. If, during a halt, the tumor returns to the irradiation range prior to this time expiring, beam output restarts. If not, the synchrotron switches to deceleration when the time expires. This operating practice enables particle beam output to continue with high irradiation efficiency if the tumor quickly returns to the irradiation range. When the Hokkaido University research team then estimated the irradiation efficiency of this new control technique, they found that it was sufficient for clinical use with a result of 43% or higher. The joint development encompassed not only the combination of different technologies, but also set high targets for itself, making a point of minimizing the treatment time in response to the strong desire expressed by clinical staff for the stress on patients to be kept to a minimum. By shortening treatment times to a few minutes or a few tens of minutes, lifting irradiation efficiency from 4% to 43% significantly reduces the impact of therapy on the patient.
Figure 3—Comparison of Different Accelerator Operating Practices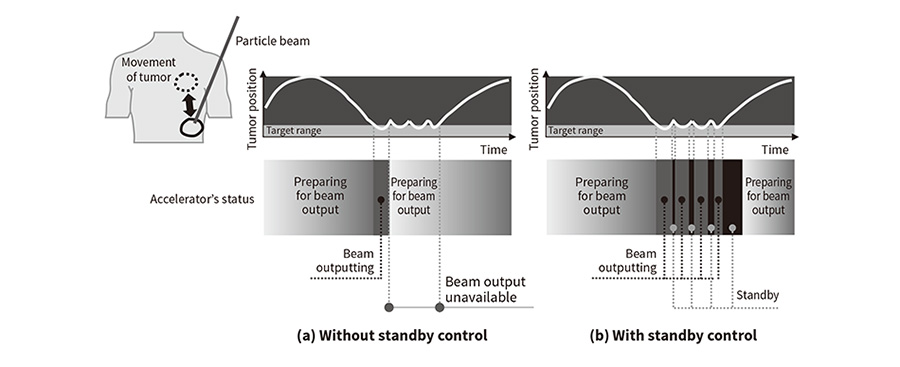 (a) The previous technique without standby control was only able to expose the patient to the beam for short periods of time because preparation for beam output restarts as soon as the tumor moves outside the target range.
(a) The previous technique without standby control was only able to expose the patient to the beam for short periods of time because preparation for beam output restarts as soon as the tumor moves outside the target range.
(b) The length of time the patient is exposed to the beam was increased by adding standby control, which is invoked when the tumor moves outside the target range.
An analysis was made of the new control technique to assess the distribution of dose, which is shaped to match the tumor. A target, a 6-cm cube used to replicate the tumor, was placed in water used to replicate a human body. The conditions for achieving a uniform dose in the target were then determined. Assuming that the movement of the target would follow a sine wave function raised to the fourth power with a peak-to-peak amplitude of 20 mm and a period of 3 s, the range of positions within that movement in which irradiation would be enabled was specified and a study was made of the relationship between the amplitude and the degree of uniformity of dose distribution within the target. Three directions of movement were specified: parallel to the preferred scanning direction for spot irradiation (primary direction), perpendicular to this direction (secondary direction), and the depth direction (direction of the proton beam) [see Figure 4(a)]. The results found that the desired dose uniformity (within 3% of the intended value) can be achieved regardless of the direction of target movement for an irradiation range of 2 mm or less [see Figure 4(b)]. Moreover, to conduct testing under more realistic conditions, the Hokkaido University research team undertook a study using data on the respiratory movements of actual patients treated using the previous tumor-tracking radiation therapy technique, which used X rays, and computed tomography (CT) data used for treatment planning. The study found that use of tumor tracking and spot scanning in combination can achieve precise irradiation of the tumor by using irradiation ranges of ±2 mm(7), (8). As these studies demonstrated that both irradiation efficiency and dose distribution are suitable for clinical applications, the new control technique was adopted.
This work has been recognized as a notable example of collaboration between a university and company, having won the Ministry of Education, Culture, Sports, Science and Technology Award at the 13th Industry-Academia-Government Collaboration Contributors Awards Program, the CyPos Presentation Award at the 101th Scientific Meeting of the Japan Society of Medical Physics, the Judge Committee's Special Prize at the 44th Japan Industrial Technology Awards, and the Science, Technology, and Economics Committee Chairman's Prize at the 6th Technology Management and Innovation Awards.
It is not possible for medical practitioners or company researchers to develop medical devices on their own. Likewise with this project, the development of the new tumor tracking technique came about through testing based on the practical input and clinical data of the Hokkaido University research team and the technical development capabilities of Hitachi that had been built up by those who had worked on this technology in the past, and by combining and merging the respective strengths of both parties.
Figure 4—Results of Dose Distribution Testing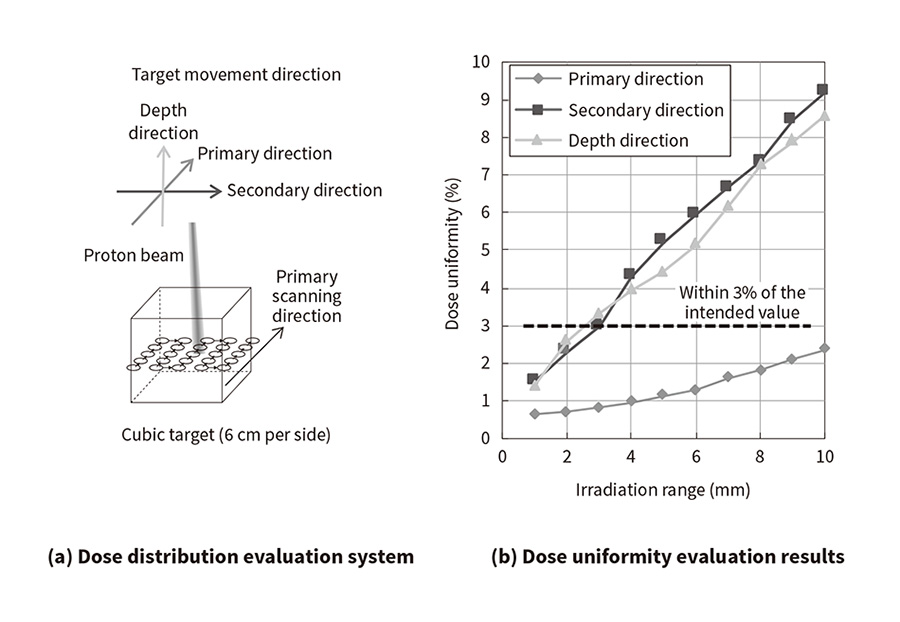 (a) The dose distribution was obtained for a
cubic target that moved in accordance with a mathematical function that replicated respiratory movement.
(a) The dose distribution was obtained for a
cubic target that moved in accordance with a mathematical function that replicated respiratory movement.
(b) Although there is some variation depending on the scanning direction, the target for dose uniformity (within 3% of the intended value) can be achieved for an irradiation range of 2 mm.
During the development, the importance of intellectual property was brought up with the Hokkaido University research team. To this end, meetings for coming up with ideas were held once every three months during the course of the Funding Program for World-Leading Innovative R&D on Science and Technology. Hokkaido University and Hitachi jointly applied for 10 patents for inventions that arose out of the industry-academia collaboration during the development. These included those relating to the new control technique described in this article and the imaging X-ray and proton beam output timing, and also a tracking technique that changes the proton beam position in a way that follows the position of a target, an approach that has future potential. Of these eight were granted in Japan and four overseas.
The patent for the tumor tracking invention was granted in March 2016, with Hitachi's joint entry with Hokkaido University in the FY2017 National Commendation for Invention being made at the same timing. These developments and associated intellectual property were subsequently recognized by being awarded the Imperial Invention Prize, the top prize at the FY2017 National Commendation for Invention. This was the first such achievement by Hitachi in approximately 20 years.
This article has described work with Hokkaido University on the development of a technique for precisely targeting a proton beam at tumors that move due to respiration and other bodily functions. This involved the development of an irradiation method that combines spot scanning with the tumor tracking technique developed by Hokkaido University, and included coming up with synchrotron operating practices that improve irradiation efficiency. The intention for the future is to contribute to the wider adoption of particle beam therapy by continuing with work on extending the application of the techniques developed through this research to particle beam therapy systems that use carbon ion beams as well as those that use proton beams.
The work described in this article was undertaken under the Advanced Radiation Therapy Project: Real-time Tumor-tracking with Molecular Imaging Technique through Japan's Funding Program for World-Leading Innovative R&D on Science and Technology, a scheme designed by the Cabinet's Council for Science and Technology Policy. The work was the result of joint research with Professor Hiroki Shirato, Faculty of Medicine, Hokkaido University, Professor Kikuo Umegaki, Faculty of Engineering, Hokkaido University, and their research group. The authors would like to thanks everyone involved in this research and development for their advice and support.