Same-day testing and same-day diagnosis have become widespread practices at medical institutions over recent years, contributing to improvements in QoL and healthcare quality by reducing the hospital visits of patients and rapidly identifying optimal treatments from data. Hitachi High-Tech Corporation supplies clinical chemistry and immunochemistry analyzers that support this work. Ongoing product enhancements include the automation of consumables replenishment and maintenance to cover operation at peak times or late-night emergency testing, and anomaly detection methods that enhance test result reliability. For the future, Hitachi also intends to continue expanding the scope of rapid testing through the adoption of new detection techniques and advanced information technologies.



It is estimated that around 70% of treatment plans in healthcare are decided on the basis of in vitro diagnostics(1). The environment surrounding medical care has brought about breakthroughs in cancer treatment and intractable diseases through genome-wide analysis and the advent of antisense drugs and antibody drugs. The emergence of new expensive and risky therapeutic techniques has created a need for improvements in both the quality and speed of therapy selection. In the field of in vitro diagnostic systems, the ISO 15189 standard (Medical laboratories — Requirements for quality and competence) was established out of a desire for guaranteed results to facilitate knowledge acquisition and rapid therapy selection. At the clinical laboratories where this work is done, there has been a notable increase in urgency around areas like compliance with international standardization and ensuring rigor in quality control and equipment maintenance practices. Medical institutions on the front line of healthcare are seeking to put practices in place to ensure that the results of in-vitro diagnostics are provided in fixed time. Motivating this is that same-day testing and same-day diagnosis make it possible to rapidly narrow-down what a patient is suffering from and to identify therapies based on detailed information about their condition, with benefits that include improving quality of life (QoL) for patients and their families as well as cutting costs. Clinical chemistry and immunochemistry testing represents one area of in vitro diagnostics where testing is fast enough to be done while the patient waits (taking roughly 15 minutes for pre-treatment and 10 to 30 minutes for the test itself). If the analyzer is out of action due to maintenance or the replacement of consumables, the test sample will be held up at the clinical laboratory and the waiting time for the patient will be longer. Accordingly, Hitachi High-Tech Corporation has dedicated itself to developing new products that provide medical institutions with uninterrupted testing.
Figure 1 — cobas pro Integrated Solutions System
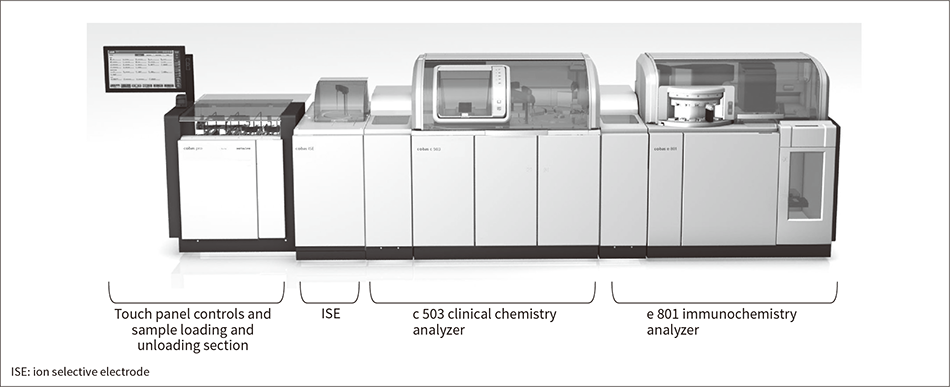 The photograph shows an integrated system made up of an ISE module, c 503 clinical chemistry analyzer, and e 801 immunochemistry analyzer. Depending on the particular laboratory requirements, the system can also be configured with just an ISE module and c 503 or e 801. The ISE module has a capacity of 300 samples/hour and the c 503 and e 801 can perform 1000 and 300 tests/hour respectively. The system is primarily used in medium- and large-sized hospitals.
The photograph shows an integrated system made up of an ISE module, c 503 clinical chemistry analyzer, and e 801 immunochemistry analyzer. Depending on the particular laboratory requirements, the system can also be configured with just an ISE module and c 503 or e 801. The ISE module has a capacity of 300 samples/hour and the c 503 and e 801 can perform 1000 and 300 tests/hour respectively. The system is primarily used in medium- and large-sized hospitals.
Hitachi High-Tech led the world in bringing integrated automatic analyzers for clinical chemistry and immunochemistry testing to market and remains a leader in the field. Clinical chemistry and immunochemistry analyses involve identifying the substances present in biological fluids, especially blood samples. The results are used for purposes such as diagnosis, therapeutic drug selection, and assessing treatment effectiveness. As an example of this, the following section describes the latest cobas* pro integrated solutions system for clinical chemistry and immunochemistry analysis that was developed in collaboration with Roche Diagnostics to support same-day testing and same-day diagnosis with the aim of delivering integrated solutions that provide even more reliable test data and enable samples to be accepted 24 hours a day. cobas pro is an integrated system made up of an ion selective electrode (ISE) module, cobas c 503 clinical chemistry analyzer, and cobas e 801 immunochemistry analyzer that are able to operate in series (see Figure 1).
The following sections describe two key features of the system: how cobas pro automates maintenance and consumables replenishment and its anomaly detection capability.
Figure 2 — Typical Operation at Existing Hospital Laboratory
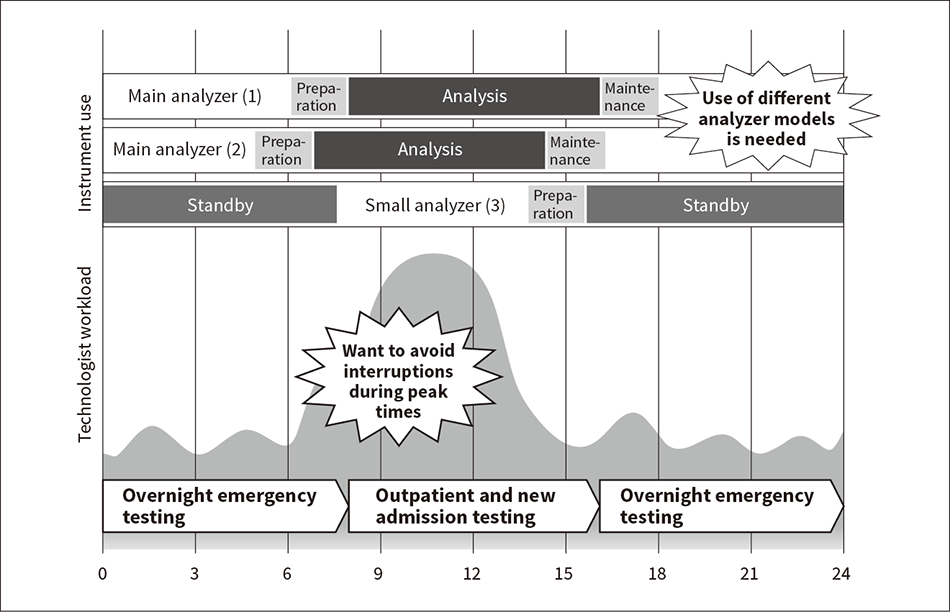 The peak in incoming blood testing samples usually occurs in the morning when the bulk of outpatient and new admission samples are taken. A number of analyzers operate in parallel to provide the required processing capacity. Testing can continue without interruption by offsetting the timing of preparation, analysis, and maintenance on each analyzer.
The peak in incoming blood testing samples usually occurs in the morning when the bulk of outpatient and new admission samples are taken. A number of analyzers operate in parallel to provide the required processing capacity. Testing can continue without interruption by offsetting the timing of preparation, analysis, and maintenance on each analyzer.
Standard practice in the past at medical institutions that operate 24-hour testing has been to use a number of high-throughput systems during the day and smaller systems at night, and to operate a variety of different analyzer models (see Figure 2). It is done this way so that maintenance can be performed on primary testing systems during the evening or overnight. Because the required daily maintenance and the replacement of empty reagent packs both involve shutting down the analyzer, it is undesirable for such shutdowns to happen during peak testing times. This means that laboratory technologists need to become familiar with the use and maintenance of a number of different analyzer models.
Providing 24-hour-a-day sample processing with analyzers able to operate continuously directly reduces the amount of time laboratory technologists need to spend dealing with their equipment. That is, the cobas pro system brings a major reduction in laboratory technologist workload.
Figure 3 — Reagent Autoloader and Reagent Packs
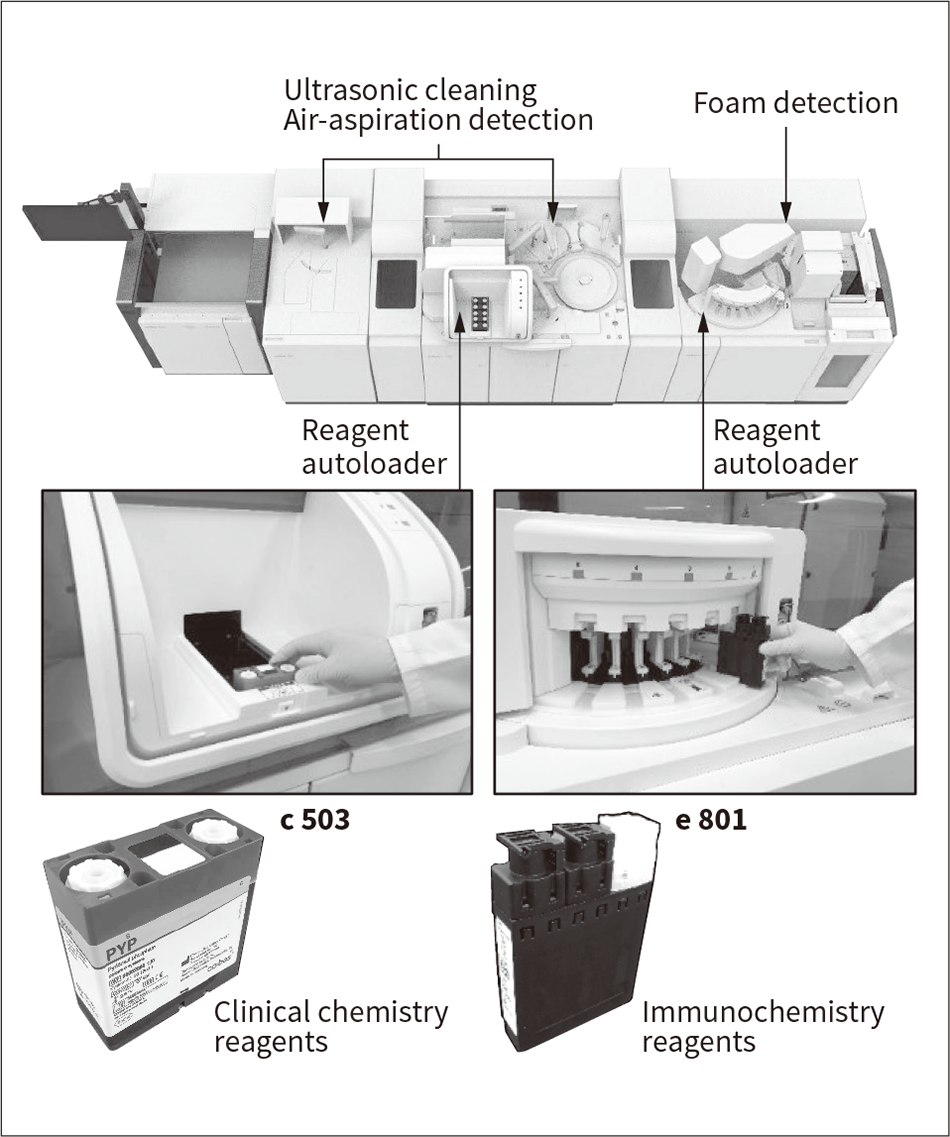 The photograph at the top shows the top cover open and the reagent autoloader visible. The lower photographs show the reagent autoloader and reagent packs for the two analyzers.
The photograph at the top shows the top cover open and the reagent autoloader visible. The lower photographs show the reagent autoloader and reagent packs for the two analyzers.
cobas pro is equipped with reagent autoloaders on both the c 503 and e 801 analyzers that allow laboratory technologists to replenish reagent packs whenever they want. Typically, several dozen reagent packs are kept under refrigerated conditions in the reagent disk of the analyzer. The reagent disk works by rotating to move various reagent packs to a reagent pipetting position as required during analysis. When the amount of reagent remaining in a pack runs low, the laboratory technologist loads a replacement. On past analyzers, this required the technologist to interrupt analysis operation to halt the movement of the reagent disk for the reagent pack to be replaced. Furthermore, differences in type and amount consumed for the reagent packs used in clinical chemistry analyses and immunochemistry analyses mean these packs have different shapes, while different pipetting nozzle cleaning methods mean that they also differ in how they are uncapped. On the cobas pro in contrast, both analyzers have reagent autoloaders that allow the various different reagent packs to be loaded without any uncapping or other preparatory steps by the laboratory technologist (see Figure 3).
The reagent packs loaded into the reagent autoloader are automatically transferred to the reagent disk at a time when this will not interfere with other mechanisms. This means that laboratory technologists are able to load reagent packs when it suits them, without halting analysis. At this time, reagent packs that are running low are automatically ejected to the loading section of the reagent autoloader. This puts the laboratory technologist in control of the workflow, allowing them to replenish reagent packs when it suits them, regardless of the current progress of analysis. Meanwhile, factory testing at the cobas pro manufacturing plant uses a robot for automatic loading of reagent packs. The deployment of such robots at clinical laboratories has the potential to deliver further workflow improvements. Likewise, system development has included improvements aimed at making each reagent pack last longer. In the case of clinical chemistry analysis, this involved use of concentrated reagents and provision of a function for diluting these. For immunochemistry analysis, reagent storage temperature was optimized and the time caps stay open was shortened. Furthermore, use of radio frequency identification (RFID) ensures reliable management of expiry dates and remaining reagent levels for the reagent packs loaded into the system, eliminating the need for manual data entry by technologists. In this way, cobas pro can help reduce laboratory technologist workloads at large laboratories and commercial laboratories in particular.
Figure 4 — Automation of Daily Maintenance
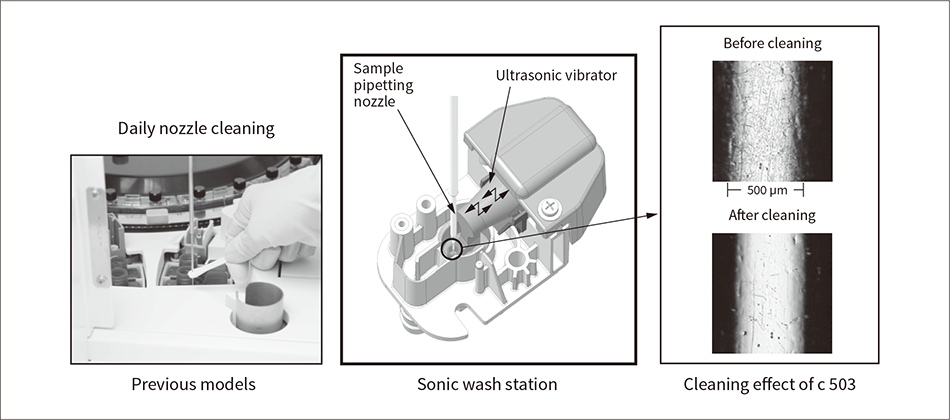 Standard practice on past analyzers has been to clean nozzles daily using a cotton swab or similar (left). On the ISE module and c 503, ultrasonic cleaning of the sample pipetting nozzles is performed automatically at regular intervals during operation.
Standard practice on past analyzers has been to clean nozzles daily using a cotton swab or similar (left). On the ISE module and c 503, ultrasonic cleaning of the sample pipetting nozzles is performed automatically at regular intervals during operation.
Past automatic analyzers have required maintenance after use such as cleaning sample pipetting nozzles. On the c 503, the system performs such routine maintenance automatically during operation. This eliminates the need for user action and the analyzer appears to carry on performing analyses. However, in the case of reaction cell cleaning, for example, a task that needs to be performed weekly, cleaning all 221 cells at the same time would require the analyzer to stop processing samples for about 30 minutes. Instead, this halt in testing can be prevented by programming the system to spread this cleaning task across five days and to perform it at times when there are no samples to process.
To help automate maintenance, Hitachi also developed a miniature ultrasonic cleaning unit for the sample pipetting nozzles. Whereas past practice was to halt the analyzer each day to wipe the nozzles clean, this task has been eliminated by automatically performing ultrasonic cleaning at fixed intervals during system operation (see Figure 4).
Eliminating the need to halt the system for maintenance each day frees laboratory technologists from this routine maintenance work and also helps prevent mistakes by inexperienced staff such as bending nozzles during cleaning.
Two causes of erroneous data in sample testing due to inability to aspirate and dispense the required quantity of sample are: (1) nozzles being clogged by fibrin or other solid matter contained in blood samples, and (2) foam on the surface or insufficient sample quantity. Investigating and taking action in response to such data problems takes up laboratory technologists’ time. A technique used to identify cases of nozzle clogging is to extract feature values from the pressure waveform for sample aspiration and use the Mahalanobis distance to detect clogging problems. Unfortunately, this does not work as well for the latter problem of insufficient sample aspiration caused by aspiration drawing in air rather than sample material as in this case the difference from the normal pressure waveform is smaller than that for clogging. To further improve the reliability of testing data from the cobas pro, the c 503 and e 801 analyzers have both been equipped with detection techniques that suit their respective characteristics.
The c 503 features a new algorithm for detecting when air aspiration, as follows.
To obtain the coefficient vector for the scoring formula, teaching data was acquired by measuring pressure waveforms for a mix of successful and unsuccessful sample intakes on a number of analyzers using samples with different viscosities and other properties. Logistics regression, a form of generalized linear model (GLM), was then used to determine the coefficients. An alarm appears in the results field when the algorithm indicates that aspiration has failed to draw in sufficient sample.
The e 801, meanwhile, includes a function that uses image analysis to detect foam in samples. A camera mounted above the sample container captures an image of the sample surface prior to drawing fluid for testing. This image is used in an algorithm to determine whether or not foam is present on the sample surface. If so, the system suspends sample pipetting and instead alerts the technologist to the problem. In developing this function, Hitachi High-Tech used convolutional neural network (CNN) machine learning to formulate the algorithm on the basis of more than one million sample images, using samples with four different colors and in five different containers captured in more than 15 different ways.
These detection functions ensure that test results are more reliable and reduce the amount of effort laboratory technologists need to put into checking for problems with foam or sample intake.
Whereas the above sections have described the existing functions of cobas pro, this section looks ahead to technologies likely to be used in the future in vitro diagnostic systems.
Advances in big data analytics and data communications have facilitated a shift away from conducting maintenance on the basis of simple measures such as operating time or number of tests processed, basing it instead on actual use. Analyzer systems collect a wide variety of data, including measurement data, operation logs, and maintenance records. By collating this data remotely on an upstream information system and applying machine learning or other forms of artificial intelligence (AI), and by combining this with information held by the system vendor, it is possible to predict what maintenance work should be done and when best to do it. One possibility would be to have the upstream system monitor parameters that are correlated with fatigue in a particular component so as to recommend replacement when it reaches a predetermined level. The choice of parameters does not need to be limited only to operating time or number of tests processed, and can change to take account of things like how the customer uses the analyzer and what sort of tests they perform. This means that, based on these changes, the upstream system is able to plan maintenance in accordance with actual usage. The knowledge of analyzer usage acquired from data analysis can also be utilized to improve existing systems or in the development of the next generation of systems.
Progress is being made on the IT-based sharing of test data as a means of coordination between different healthcare facilities, the cross-border sharing of clinical trial data, and the use of anonymized big data in medical research. An essential requirement for the remote sharing of data is the standardization of test results to ensure consistency in sample measurement regardless of which facility conducted the testing. In clinical chemistry and immunochemistry analysis, reference values and test results typically differ between country, region, and facility due to differences in reagent formulations or in the equipment combinations and measurement environment. Progress is being made on the standardization of testing around the world through measures that include uniform formulations for reagents and the ISO 15189 accreditation of clinical laboratories. Standardization provides access to a timeline of patient data in situations such as when they are transferred from their general practitioner to a specialist clinic, or from an emergency department to a facility that offers rehabilitation services, for example. The requirement for analyzers, then, is a high level of reliability in terms of results with low variability and not being influenced by their specific environment.
To ensure that its systems are able to deliver highly reliable data wherever in the world they are used, Hitachi High-Tech conducts electromagnetic compatibility (EMC) testing and testing under low pressure conditions (to allow for use at high altitude), and complies with the cybersecurity requirements of the US Food and Drug Administration (FDA). By doing so, it is helping to provide test result portability through the use of IT.
Figure 5 — High-sensitivity Mass Spectrometry (New Type of Ion Guide)
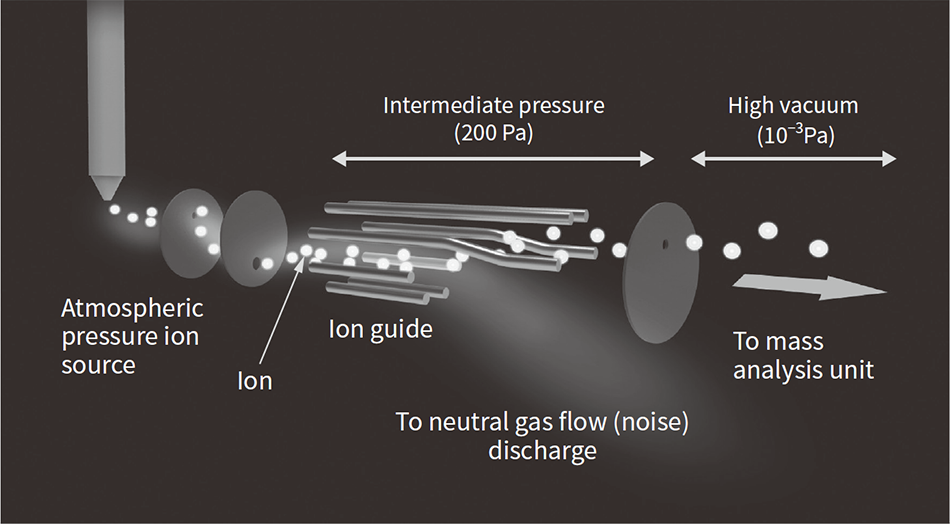 To improve long-term stability, the instrument also includes a function for eliminating the neutral gas that, as well as serving as a medium for the deflection and efficient transmission of ions, also interferes with measurement.
To improve long-term stability, the instrument also includes a function for eliminating the neutral gas that, as well as serving as a medium for the deflection and efficient transmission of ions, also interferes with measurement.
Mass spectrometry is a technique for ionizing the components to be measured at the molecular level and measuring them separately based on their respective molecular weights, and makes it possible to detect ions with high sensitivity and high selectivity compared to other measurement techniques. It also has a high level of affinity with liquid chromatography (a separation technique), such that the two techniques used together are a recognized means of obtaining comprehensive measurements of metabolomics analysis on proteins, other biological digestives, and substances such as vitamin D and therapeutic drugs in blood(2). Because of these excellent characteristics, mass spectrometry has recently come to be widely used in clinical tests mainly in Europe and the USA.
On the other hand, in order to use a mass spectrometer in a laboratory, there are still problems in stable operation and automation of a high-sensitivity system for a long period of time, and it requires a specialist operator who is familiar with mass spectrometry technology at present. To address these problems, a new type of ion guide technology that contributes to the stable operation of a high-sensitivity system has been introduced (see Figure 5). In a mass spectrometer, ions derived from a measurement object generated in an ion source at atmospheric pressure are detected by a spectrometer installed in a high vacuum (10-3 Pa: one-hundred-millionth of atmospheric pressure). In order to obtain high sensitivity, it is necessary to introduce ions generated at atmospheric pressure into the mass spectrometer with high efficiency, and on the other hand, for stable operation, it is necessary to have a mechanism for not introducing neutral gas as a pollution source into the mass spectrometer. In order to meet these conflicting requirements, a specially shaped ion guide was installed in the intermediate pressure region (approximately 200 Pa) to deflect ions and allow them to pass through efficiently while at the same time removing neutral gas as a pollution source(3). It is hoped that the development of these new technologies will provide reliable ways of overcoming the practical challenges of mass spectrometry and encourage greater clinical use in the future.
The introduction of same-day testing and same-day diagnosis is leading to greater use of in vitro diagnostics in healthcare. The ability to formulate treatment plans on a same-day basis is of great importance when it comes to improving QoL for patients and their families. To facilitate medical practices that are predicated on the availability of test results, it is essential that these results be delivered without delays. Meanwhile new types of tests and new testing systems are being developed along with advances in drugs and medical technology. This in turn is increasing the workload on the laboratory technologists who carry out these tests. In response, Hitachi High-Tech intends to equip cobas pro with new automation practices and reliability improvements aimed at ensuring uninterrupted operation in the core clinical chemistry and immunochemistry analysis functions of medical testing.
In the future, the need for more precise diagnosis and therapy selection will create demand for systems that are able to take a wider range of measurements from a single sample. While around 300 tests are currently available, there is a strong need to expand the scope of rapid testing by increasing this number. Hitachi High-Tech intends to continue developing new testing techniques and delivering them seamlessly while also contributing to advances in medical systems through the integration of IT and AI.