Information contained in this news release is current as of the date of the press announcement, but may be subject to change without prior notice.
November 12, 2015
Verified battery operation at the temperature as high as 150℃ with a discharge of
90% of theoretical value by internal resistance to enhance charge-discharge
performance
Tokyo, November 12, 2015 --- Hitachi, Ltd. (TSE: 6501) and Tohoku University's Advanced Institute for Material Research(AIMR) have developed a basic technology to reduce the internal resistance of the all-solid-state lithium ion battery (Li-ion battery) using a complex hydride*1 as a solid electrolyte. The reduction of internal resistance improves the charge-discharge performance of the all-solid-state Li-ion battery, resulting in the batteries (capacity: 2 mAh) successfully operating at temperatures as high as 150℃ with a discharge capacity of 90% of theoretical value*2. This technology is significant as it allows the thermally durable Li-ion battery to be used in a wider variety of applications, such as large-scale industrial machines with motors, and medical machines which need to be heated for autoclave sterilization. Since this technology does not require the cooling system common in conventional Li-ion batteries. It is expected to lead to further developments compact battery systems and reduce the overall costs.
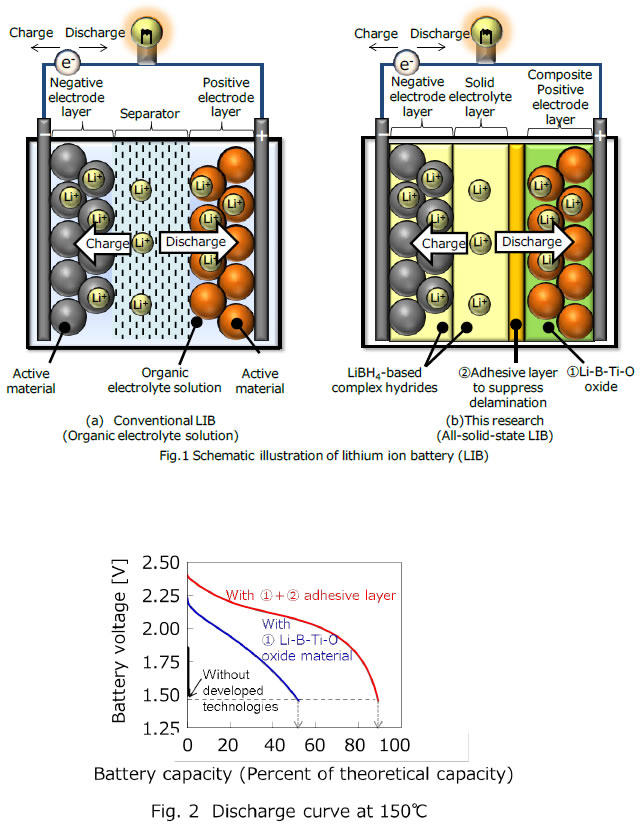
The high energy density Li-ion battery is already being used as power sources in applications such as portable devices (smartphone and tablet), electric vehicles and adjustor of the supply and demand of renewable energy. The conventional Li-ion battery consists of a separator*3,a positive electrode layer, and a negative electrode layer (Fig.1(a)). The battery is filled with organic electrolyte solution in which lithium ion conducts between the two electrode layers during the charge and discharge process. An issue of the conventional Li-ion battery with the organic electrolyte solution is thermal durability. The upper operating temperature was limited to around 60℃ owing to volatility of the organic electrolyte solution. Consequently, it is difficult to use the conventional Li-ion battery in a high temperature environment without a cooling system. Therefore, the solid electrolyte with no volatility has been developed for the utilization of Li-ion battery in a high temperature environment. The lithium ion conductivity of solid electrolyte, however, is lower than that of the organic electrolyte solution, and the internal resistance of all-solid-state Li-ion battery should be reduced for its commercialization.
Prof. Shin-ich Orimo's lab in AIMR and the Institute for Material Research at Tohoku University have been conducting research on LiBH4-based complex hydrides as novel solid electrolytes. They have confirmed the fast lithium ion conductivity in the wide temperature range from room temperature to 150℃. This research was part of a collaborative project between Hitachi and AIMR has developed the new technology to reduce the internal resistance that is a factor of deterioration of charge-discharge performance. This new technology was validated to yield the battery operation at a temperature as high as 150℃.
Details of the technology developed are as below:
One of the issues is that Li-ion conductivity will be inhibited from the decomposition of positive electrode material reduced by LiBH4 based complex hydrides. To solve this issue, Li-B-Ti-O based oxide material was developed to form the dense composite positive electrode with the active materials(Fig.1(b)①). The Li-B-Ti-O in the electrode effectively protected the active materials, and suppressed the increment of internal resistance caused by the decomposition. In consequence, the discharge capacity*5 of the battery was improved from 0 to 50% of theoretical value (Fig.2).
The other issue is that composite positive electrode layer and the metal hydride complex solid electrolyte layer were delaminated owing to the volume change of the active materials during charge-discharge reaction. This causes the increment of the interfacial resistance by poor lithium ion conduction at the delaminated interface. Therefore, in order to prevent delamination of the interface, amide-added metal hydride complex with a low melting point was developed and placed in the developed battery as an adhesive layer (Fig.1(b)②). As a result, the internal resistance of the all-solid-state Li-ion battery was successfully reduced to 1/100 of the value in the case of no adhesive layer. The discharge capacity was improved up to 90% at 150℃ by applying the developed technologies of 1. composite positive electrode layer and 2. adhesive layer (Fig.2). In addition, the degradation of the discharge capacity during charge-discharge cycles was effectively suppressed and the stable charge-discharge of the all-solid-state Li-ion battery was confirmed.
In this research, the battery operation in a high temperature environment of 150℃ with a discharge capacity of 90% of theoretical value was confirmed from a prototype of Li-ion battery with the capacity of 2 mAh and the energy density of 30 Wh/L. These are equivalent to 1/1000 and 1/20 of a Li-ion battery used in smartphone. This investigation has verified the fundamental operation of a thermally durable all-solid-state Li-ion battery, and, for practical use, we intend to look into further improving battery capacity, energy density and charge-discharge duration.
This research was part of a project between Hitachi and AIMR called "Collaborative Research for Next Generation Innovative Battery". The findings of this research will be partially presented on 13th November at The 56th Battery Symposium, which will be held in Aichi Pref. from 11th to 13th November, 2015.
Hitachi, Ltd. (TSE: 6501), headquartered in Tokyo, Japan, delivers innovations that answer society's challenges with our talented team and proven experience in global markets. The company's consolidated revenues for fiscal 2014 (ended March 31, 2015) totaled 9,761 billion yen ($81.3 billion). Hitachi is focusing more than ever on the Social Innovation Business, which includes power & infrastructure systems, information & telecommunication systems, construction machinery, high functional materials & components, automotive systems, healthcare and others. For more information on Hitachi, please visit the company's website at http://www.hitachi.com.
The Advanced Institute for Materials Research (AIMR) at Tohoku University is one of nine World Premier International Research Center Initiative (WPI) Programs established with the support of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT). The program aims to develop world-class research bases in Japan. After its establishment in 2007, AIMR has been active in conducting research activities and creating new systems in order to become a global center for materials science. Since 2012, AIMR has also been conducting fundamental research by finding connections between materials science and mathematics.
Learn more at http://www.wpi-aimr.tohoku.ac.jp