Increases number of charge/discharge cycles by up to 60% to reduce life-cycle cost of battery energy storage systems
April 19, 2021
To reduce the lifecycle cost of lithium-ion batteries (LIBs), a key component of the decarbonized society, Hitachi, Ltd. has developed an organic solid electrolyte (Fig. 1) featuring both less volatility and chemical durability using materials informatics*1 (MI) technology and has successfully fabricated a long-life LIB prototype using this electrolyte. In a charge/discharge cycle test*2, this LIB exhibited an increased number of charge/discharge cycles by up to 60% (extending battery lifetime by approximately 1.6 times) compared with LIBs using a conventional organic electrolyte. Going forward, Hitachi aims to work with some partners to make LIBs using this electrolyte practical, to apply them to battery energy storage systems, and to popularize their use. Hitachi will also promote the use of this technology to realize electrification of various mobility such as automobiles and trains and stabilize power grid systems linked to renewable energy toward the creation of a decarbonized society. Some part of this research received the Technology Award (Tanahashi Award) of the Electrochemical Society of Japan as "Development of Thermally Durable Electrolyte for Lithium-ion Batteries."
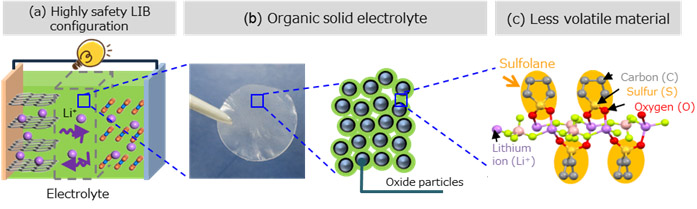
Fig. 1 Overview of long-life organic solid battery
A small-sized LIB was fabricated using the developed less volatile electrolyte and the number of charge/discharge cycles could be increased by up to 60% compared with LIBs using a conventional organic electrolyte.
Some of this research were presented at the 88th ECSJ Spring Meeting held online on March 23rd, 2021 as the award lecture for the Technology Award (Tanahashi Award) of the Electrochemical Society of Japan.
This research was partially supported as part of a commissioned project [JPJ004596] under the Innovative Science and Technology Initiative for Security of the Acquisition, Technology & Logistics Agency (ATLA).
The lithium-ion conductive liquid used in organic solid electrolytes consists of a concentrated solution composed of a less volatile solvent and lithium salt to ensure safety, and a low-viscos solvent to promote smooth charge/discharge reactions. The advanced analysis on a LIB using a previously developed organic solid electrolyte revealed that that the less volatile solvent (tetraglyme, Fig. 2 (a)) and the low-viscosity solvent (propylene carbonate) would decompose on the surface of electrodes during charge-discharge cycles thereby degrade battery capacity.
Looking to increase the durability of the less volatile solvent deemed to be a factor in this degradation, we selected a candidate material using MI technology. Targeting a solvent group with high volatilization temperature, we computed chemical stability parameters using molecular orbital calculations and extracted electrolyte materials for which high durability could be expected compared with previously developed material.
In collaboration with researchers at Yokohama National University (YNU), we developed an organic solid electrolyte using sulfolane (Fig. 2 (b)), one of the electrolyte materials extracted in the above development. Focusing on Li-ion hopping conduction between molecules in sulfolane as discovered by YNU, we applied this feature to an organic solid electrolyte and confirmed the selective conduction of Li ions. As a result, we successfully designed and prototyped an organic solid electrolyte that reduced the low-viscosity solvent by 60% while maintaining the high conductivity of the Li ions.
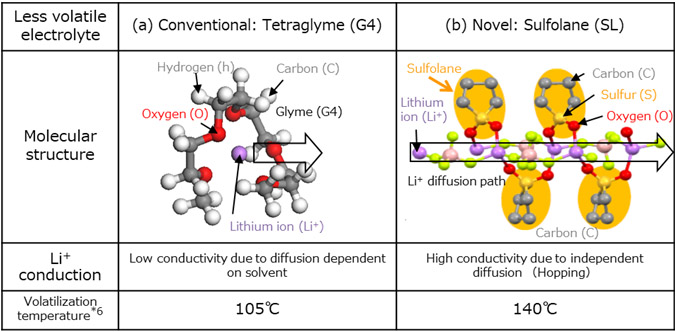
Fig. 2 Schematic diagrams and features of old and new electrolytes
On applying the developed organic solid electrolyte described above to a LIB, we found that the capacity retention during charge/discharge cycles improved and that the new battery showed promise in achieving a 60% increase in cycle lifetime compared with other organic electrolyte batteries evaluated by Hitachi. In addition, volatilization temperature of the developed electrolyte improved*6 up to 140°C from that of the conventional organic solid electrolyte (105°C), which will improve thermal durability and then safety of the LIB.
For more information, use the enquiry form below to contact the Research & Development Group, Hitachi, Ltd. Please make sure to include the title of the article.