Regenerative medicine is looking promising as a revolutionary branch of medicine that will lay the groundwork for treating underlying conditions including intractable diseases. To help foster the rise of regenerative medicine, Hitachi has adopted an open innovation approach by working with partners from academia and the pharmaceutical industry to develop automated cell culture technology for therapeutic cells. The advantage of Hitachi’s automated cell culture technology derives from it being a closed system. This article discusses the benefits of adopting closed systems to enable therapeutic cell products to be provided at a reasonable cost while guaranteeing a high degree of safety. It also looks at an intelligent automated cell culture technology that Hitachi is now working on as a way to provide cell culturing optimized for particular cell states.








Using regenerative medicine to treat a variety of intractable diseases has been researched and developed since the concept was first put forward in 1993(1). Somatic stem cells used as cell sources are currently the main practical implementation of regenerative medicine. However, the limited proliferation of somatic stem cells makes it difficult to produce cells in quantities sufficient to treat large numbers of patients. Induced pluripotent stem cells (iPS cells) were developed by a team headed by Professor Shinya Yamanaka of Kyoto University. iPS cells look promising as a solution for the shortage of cell sources needed for regenerative medicine(2). Characteristics such as a high amplification ability and pluripotency enabling differentiation into various cell types make iPS cells promising for treating a large number of diseases and helping improve patient quality of life (QoL).
The first clinical research using iPS cells was a transplant done by Japan’s Institute of Physical and Chemical Research (RIKEN) to treat age-related macular degeneration. The subject received a transplant of a retinal pigment epithelium cell sheet derived from patient’s own iPS cells(3). Cells derived from iPS cells have subsequently been used multiple times for clinical applications. They have already been used to treat Parkinson’s disease(4), for corneal cell sheet transplants(5), for transplants of myocardial cell sheets in patients with severe heart failure(6), and to treat thrombocytopenia(7). Clinical plans have been approved for treatment of spinal cord injuries(8), knee joint cartilage injuries(9), and retinal pigmentary degeneration(10). Meanwhile, amid this growth in regenerative medicine, nearly all the production of therapeutic cells is done by hand. So high production costs are still a problem. Cell mass-production enabling stable delivery to patients for a reasonable price will be a key requirement for enabling further growth of regenerative medicine. Hitachi has developed automated cell culture technology as a way to solve this issue.
This article discusses the benefits of adopting automation such as the closed-system equipment from which Hitachi’s automated cell culture technology derives its advantage. It also looks at work on future automated cell culture technology.
Figure 1 — Two Hitachi Automated Cell Culture Equipment Models
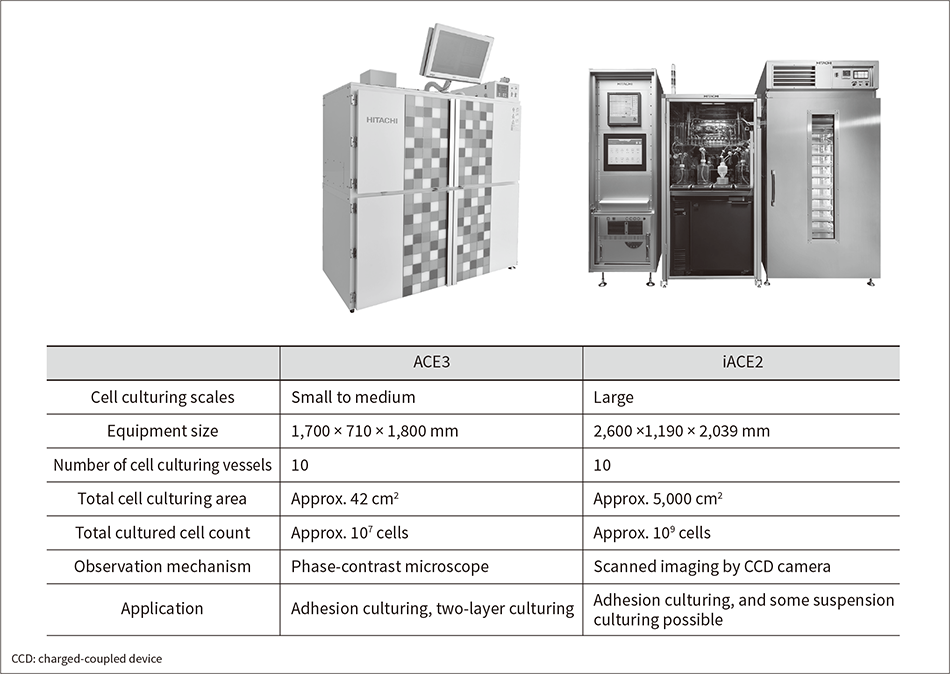 Shown here are the ACE3 model for small-scale cell culturing (left) and the iACE2 model for mass cell culturing (right).
Shown here are the ACE3 model for small-scale cell culturing (left) and the iACE2 model for mass cell culturing (right).
Hitachi started developing automated cell culture technology in FY2002. Viewing safety assurance as the top priority when producing cells for medical applications, the company has always developed closed-system automated cell culture technology that prevents microbial contamination from outside. Closed-system cell culturing uses a closed-system channel. One end of the channel has a bottle or other vessel holding the cell culture medium needed for culturing. It is connected by tubing to a cell culture vessel. Before the cell culture process begins, the closed-system channel is sterilized with gamma rays to enable the process to take place in a sterile space.
Hitachi drew on this concept to create a platform for closed-system automated cell culture technology in partnership with Tokyo Women’s Medical University. The project developed a small-scale automated cell culture equipment model (development code name: ACE3)(11), and demonstrated its ability to automatically culture corneal and oral mucosal cell sheets(12–14). A subsequent project done with RIKEN used ACE3 to work on automated culturing of retinal pigment epithelium cell sheets. It demonstrated that higher-quality cell sheets can be produced by ACE3 than by hand(15).
Concurrent with the work on the small-scale model, a large-scale automated cell culture equipment model was developed through the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST), a Cabinet Office program providing assistance for cutting-edge research and development and led by Tokyo Women’s Medical University and Osaka University. The project resulted in a completed prototype that can automatically culture the number of cells needed for myocardial regeneration (more than 109 cells)(16). This technology was used to develop an automated cell culture equipment model in partnership with Kyoto University and Sumitomo Dainippon Pharma Co., Ltd. The equipment performs the iPS cell amplification and early differentiation process into dopaminergic progenitor cells(17). It has led to the commercial release of an automated mass cell culture equipment model (iACE2)(18).
While they perform cell culturing on different scales, ACE3 and iACE2 are both closed systems with the same operating concept. They can both be used with any adhesion-culturing cells in addition to the cells previously mentioned (see Figure 1). They can also automate cell culturing processes that form cell spheroid and proliferate cells in three dimensions without causing cell adhesion(19). So these systems have been developed as fundamental technologies providing widespread support for culturing of various cell types on a range of different scales.
Figure 2 — Comparison of Closed- and Open-system Automated Cell Culture Equipment
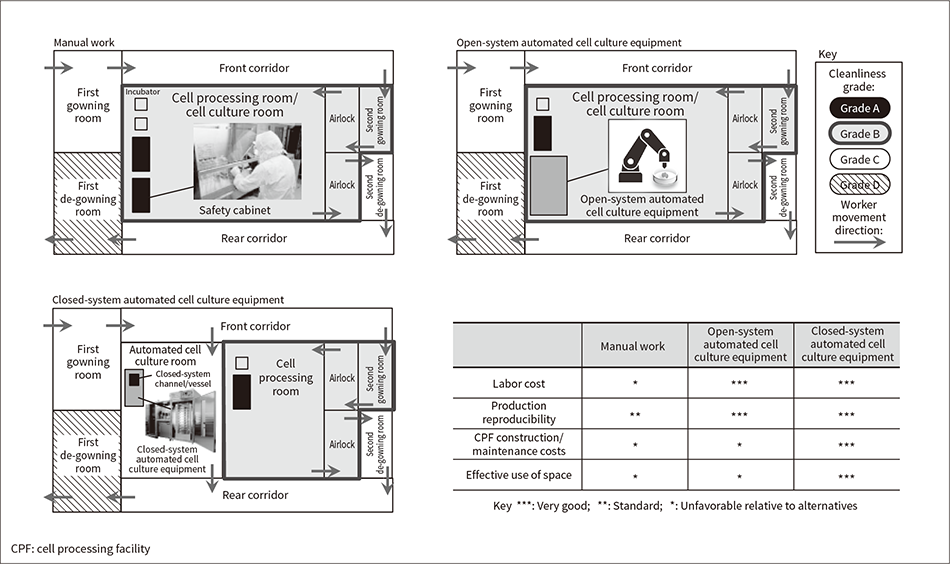 Shown here is a comparison of closed- and open-system automated cell culture equipment when installed and operated in a CPF.
Shown here is a comparison of closed- and open-system automated cell culture equipment when installed and operated in a CPF.
Therapeutic cells used in regenerative medicine are currently produced mostly by manual cell culturing done by technicians who have undergone specialized training at a cell processing facility (CPF). Meanwhile, manual cell culturing creates issues such as high labor costs, variable cell quality dependent on worker skill, and the risk of biological contamination caused by human intervention. Facility maintenance costs are also high since the work is done in spaces kept to a high standard of cleanliness. Adopting automated cell culture technology could provide an effective way to solve these issues by replacing manual work with machines. CPF operating costs are discussed below.
CPF work spaces are classified by cleanliness from Grade A (cleanest) to Grade D (least clean)(20). Work involving direct cell handling is usually done in safety cabinets with the highest cleanliness grade (Grade A). These safety cabinets are installed in cell processing rooms with highly controlled Grade B cleanliness. Workers change into gowns with guaranteed cleanliness to prevent becoming sources of biological contamination. First gowning is done when entering a Grade C room from a Grade D room, and second gowning (adding another clothing layer) is then done when entering a Grade B room. Since Grade B rooms are cleaner than Grade C rooms, they are ventilated more often and monitored more often to periodically verify that the environment is being kept clean.
Conventional work methods call for open system operations done in safety cabinets installed in Grade B rooms, and cell culturing work done in CO2 incubators. These tasks are done by specialist technicians. Various automated cell culture technologies are being developed to enable machines to take over these manual tasks. Automated cell culture equipment is classified into closed- and open-system types (see Figure 2). Closed-system equipment uses a closed channel with the cell culturing vessel, culture medium bottle, and other components connected by tubing. The cell culture space is kept very clean by keeping the system components closed until the cell culture process has finished. Open-system equipment uses the same open-system cell culturing vessels used for manual work. The open system operations are done by tools such as articulated robots. Open-system equipment components are housed in casing separated from the outside world, creating an environment that maintains cell cleanliness.
Open-system automated cell culture equipment uses open-system cell culturing vessels, so it is usually installed in a Grade B space since the vessels need to be moved outside the equipment before and after automated cell culturing. Open-system equipment contains articulated robots, so it is usually also larger than closed-system equipment. In contrast, the closed-system automated cell culture equipment being developed by Hitachi is designed for installation in Grade C spaces since it can prevent outside microbial contamination from entering through closed-system channels.
Closed-system automated cell culture equipment enables automation that lowers labor costs and improves production reproducibility. This equipment is usually smaller than open-system equipment, enabling effective use of interior space. Closed-system equipment is installed in Grade C spaces of lower cleanliness than the Grade B spaces used by open-system equipment, enabling smaller Grade B spaces. When the equipment requires some type of manual operation, fewer clothing changes are needed to enter a Grade C space than a Grade B space, lowering cost accordingly. Grade C spaces also enable lower environmental monitoring and cleaning costs for cleanliness control than do Grade B spaces. So along with automating manual work, automated cell culture equipment also offers the benefit of lower CPF construction and operation costs.
In previous work on developing automated cell culture equipment, Hitachi has cultured cells automatically by creating an automated cell culture protocol from the manual protocol. The automatic protocol has been shown to yield cells of the same quality as manually cultured cells. It was executed using a closed-system cell culturing routine equivalent to the manual procedure. This routine was programmed into the equipment and run. Next-generation automated cell culture technology determines cell states in real time and controls the culturing process through feedback. The result is an intelligent system that provides continually optimum cell culturing (see Figure 3), and can help ensure a stable and efficient supply of cells for regenerative medicine.
The first requirement for creating this type of automated cell culture equipment will be monitoring technologies used to assess cell states non-invasively and in real time. Today’s cell assessment methods are mostly invasive and time-consuming types involving cell collection and counting, or cell destruction to enable internal gene expression analysis. Hitachi has responded by starting to develop real-time non-invasive cell monitoring technology designed for installation in automated cell culture equipment(21). The ability to identify cell states in real time during automated cell culture processes will enable optimized cell cultures and help minimize loss costs by assessing whether to continue any deviant processes that may arise.
Hitachi is currently developing the monitoring technologies needed by examining cell images and components within cell culture supernatants (medium). One project is a novel attempt to measure vesicles (exosomes) released from cells in cell culture supernatants. Cell culture supernatants can be collected non-invasively when replacing the medium, making them suited to monitoring. Exosomes contain a lot of information about the inside of cells. They have attracted interest as markers for cancer diagnosis, but Hitachi is working on using them as markers for monitoring cell states during culturing. Hitachi measured the number of exosomes contained in an iPS cell culture supernatant at different stages and compared them to the number of cells present each time. The two quantities were found to be highly correlated (see Figure 4). So it may be possible to assess the cell proliferation rate from the number of exosomes in a cell culture supernatant. While this work focused on exosome counts, it should be possible to obtain more information by analyzing other exosome parameters along with internal miRNA (microRNA). Hitachi is looking to develop technology for measuring these items since they could be effective monitoring targets.
The monitoring technologies described here will make it possible to use numerical data to manage cell cultures that have been created and assessed manually. The data will provide feedback used to control the cell culture process so that it can be optimized in real time without manual intervention. Digitalizing cell production in this way will enable the use of the Digital Innovation Platform of Lumada being promoted by Hitachi. It should create new value in areas such as more efficient cell production.
Figure 3 — Conceptual Diagram of Intelligent Automated Cell Culturing
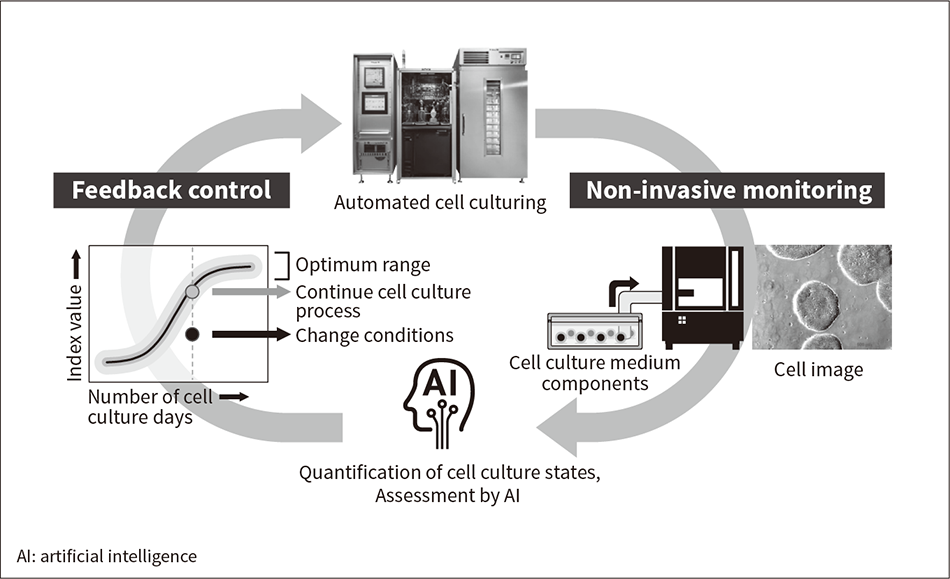 Cell states are monitored non-invasively using methods such as cell image analysis and analysis of cell culture supernatant components while culturing. Feedback is provided automatically to enable cell culturing under continually optimum conditions.
Cell states are monitored non-invasively using methods such as cell image analysis and analysis of cell culture supernatant components while culturing. Feedback is provided automatically to enable cell culturing under continually optimum conditions.
Figure 4 — Correlation between Exosome Count and Cell Count in iPS Cell Culture Supernatant
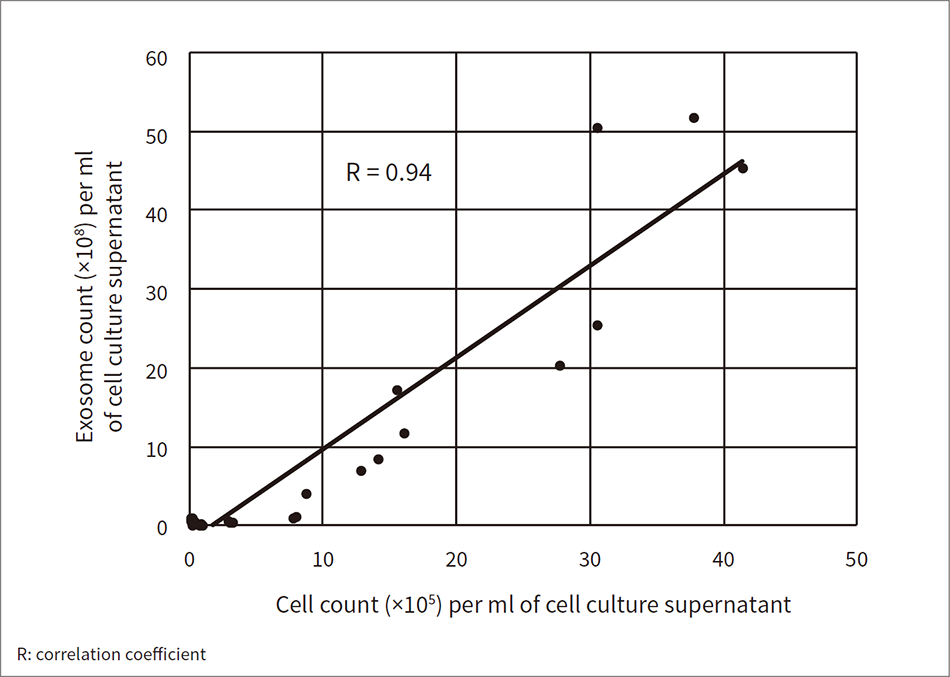 Cell culture supernatants contain exosomes (vesicles released from the cells). Tunable resistive pulse sensing was used to measure the exosome count per unit volume of iPS cell culture supernatant. The correlation between the measured exosome count and the iPS cell count was analyzed and found to be high.
Cell culture supernatants contain exosomes (vesicles released from the cells). Tunable resistive pulse sensing was used to measure the exosome count per unit volume of iPS cell culture supernatant. The correlation between the measured exosome count and the iPS cell count was analyzed and found to be high.
Automating today’s costly manual cell production processes will make it possible to produce cells at a reasonable cost. Practical implementations of regenerative medicine that use donor iPS cells will also enable many patients to benefit from effective regenerative medicine that can provide permanent cures. Autologous regenerative medicine (autologous cell transplantation) could be another increasingly promising area that enables higher patient QoL due to its very low risk of immunorejection. Cell conditions are patient-specific in autologous transplantation, so Hitachi’s intelligent automated cell culture equipment should be an effective tool for it. Since the cells to produce are tied to a single individual, traceability from sampling to transplantation will be a key requirement here.
Regenerative medicine calls for several different processes that are absent from conventional medicine. Hitachi is responding to this need by bringing a large number of Group companies together under the One Hitachi slogan to provide total solutions for regenerative medicine that could help foster the rise of this revolutionary field. The company is planning to step up the worldwide release of these solutions, starting with the first overseas release in Taiwan(22). The aim is to help make regenerative medicine available to recipients throughout the world.
Some of the work described in this article was undertaken by the following projects and organizations: (1) Development of Nano-bio Interface Technologies for Tissue Regeneration Implant (a basic technologies research promotion project of the New Energy and Industrial Technology Development Organization [NEDO]), (2) the Advanced Interdisciplinary Center for the Establishment of Regenerative Medicine (part of the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program of the Ministry of Education, Culture, Sports, Science and Technology [MEXT]), (3) System Integration for Industrialization of Regenerative Medicine: Creation of Organ Factory (the Cabinet Office’s Funding Program for World-Leading Innovative R&D on Science and Technology [FIRST Program]), (4) Grant Number JP20be0404010 of the Japan Agency for Medical Research and Development (AMED), and (5) Kobe Biomedical Innovation Cluster (KBIC) Research Grants-in-Aid for Young Researcher. The authors would also like to thank the members of Tokyo Women’s Medical University, Osaka University, the Center for iPS Cell Research and Application at Kyoto University, the Institute of Physical and Chemical Research (RIKEN), and Sumitomo Dainippon Pharma Co., Ltd. who provided guidance and assistance for the research and development work described here.