A variety of different cancer therapies have been developed and deployed in recent years. Immune checkpoint inhibitors are one such example, a type of immunotherapy that offers unprecedented long-term benefits in advanced cancer cases. Unfortunately, the response rate to these therapies remains poor, with many patients failing to benefit from them. Accordingly, in order to expand the scope of drug efficacy and increase response rate, a lot of effort is going into the development of combination therapies that use a number of different methods and into testing practices that can predict their efficacy. Hitachi is working on a promising testing technique called single-cell analysis that can measure the immune response to tumor cells with high accuracy. In place of the previous analog measurement technique using cell staining, this introduces a DNA barcode into cellular genes to serve as a digital tag and uses next-generation DNA sequencing to perform precise measurement in the form of a molecular count of the different genes. This article reports on trends in biomarker development for the development of immunotherapies and describes work toward personalized medicine using single-cell analysis.



Immune checkpoint inhibitors (ICIs) restore immune functions that have been suppressed by tumor cells. They have been touted as a cure that offers long-term benefits to some patients with advanced cancer. Unfortunately, many candidate patients receive no such benefits(1). Combination therapies that combine ICIs with other therapeutic methods and drugs are being trialed as a way to increase the response rate and expand the range of cancers for which ICIs can be used. As of September 2019, 2,949 such clinical trials were in progress around the world(2). Another active field of study is the development of biomarkers that can accurately predict drug efficacy and improve response rates by helping select which drugs to use(1). With the availability of next-generation deoxyribonucleic acid (DNA) sequencers (NGSs) having brought down the cost of sequencing, genomic information is now being put to medical use, especially in the USA. In Japan, genomic cancer therapies were authorized for coverage under the national health insurance system in June 2019(3). As the progress of cancer is the outcome of complex interactions with the immune system, there is a growing recognition of the importance not only of existing analyses for tumor cells, but also of immune cell analyses in assessing the state of the cancer and using this as a basis for appropriate treatment. In particular, the direct measurement of immune cells has shown recent promise as a means of identifying biomarkers that can be used to predict drug efficacy(1).
This article reports on what is happening with the development of biomarkers for use in ICI and other immunotherapy development, also describing work being done on using single-cell analysis for personalized medicine.
A wide variety of immune cells circulate inside the body, with the cancer-immunity cycle for recognizing and eliminating tumor cells made up of several dozen inter-cellular signaling pathways (see Figure 1)(4). Unfortunately, this cycle can also be stalled in a number of ways as the cancer progresses. Immunotherapy works by kickstarting this cycle to eliminate tumor cells using the body’s intrinsic functions. The ICIs described above reactivate immune functions that have been inhibited by tumor cells(1). Two such ICIs are anti-PD-1 and anti-PD-L1 antibodies. These work by blocking signals emitted by tumor cells to evade T lymphocytes (T cells) in the tumor cell death process (7) shown in Figure 1. By doing so, they restore the immune function provided by the T cells.
Figure 1 — Overview of Cancer-Immunity Cycle
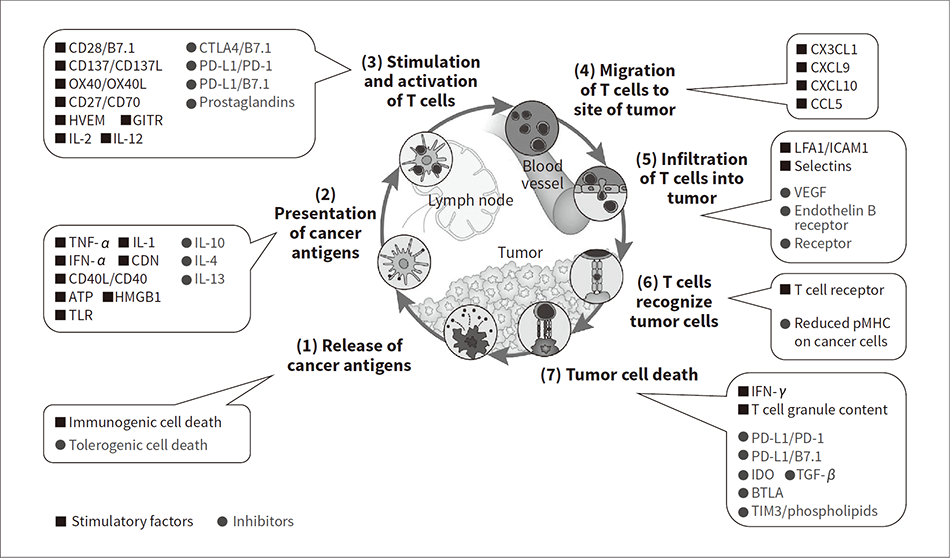 Large numbers of tumor cells are eliminated from tissue in a targeted manner by processes (1) to (7) of the cancer-immunity cycle. However, if the tumor cells can obstruct or avoid one or more of these cancer-immunity cycle processes, the tumor will continue to grow. Cancer immunotherapy works by restarting the immunological cycle.
Large numbers of tumor cells are eliminated from tissue in a targeted manner by processes (1) to (7) of the cancer-immunity cycle. However, if the tumor cells can obstruct or avoid one or more of these cancer-immunity cycle processes, the tumor will continue to grow. Cancer immunotherapy works by restarting the immunological cycle.
Figure 2 — How Use of ICIs to Treat Advanced Cancer Delivers Long-term Efficacy
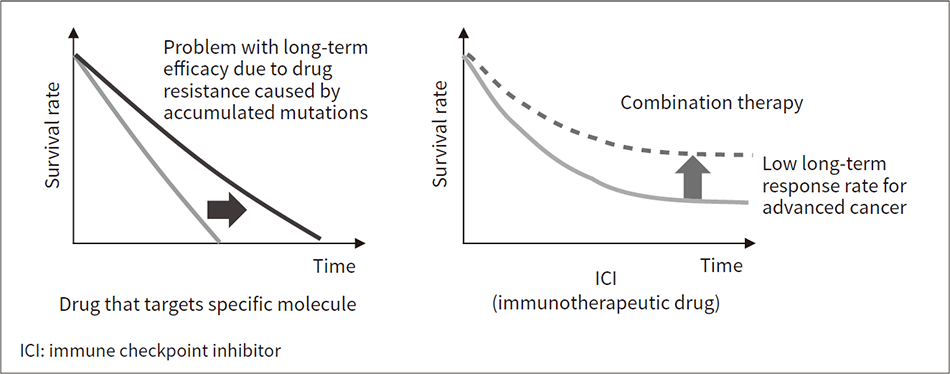 Conventional chemotherapy using drugs that target specific molecules does not deliver long-term benefits because of the loss of efficacy due to an accumulation of mutations causing drug resistance. As cancer immunotherapy works by restoring the body’s own immune functions, those therapies that do prove effective have long-lasting benefits and there is hope that use of a combination of therapies will improve response rates.
Conventional chemotherapy using drugs that target specific molecules does not deliver long-term benefits because of the loss of efficacy due to an accumulation of mutations causing drug resistance. As cancer immunotherapy works by restoring the body’s own immune functions, those therapies that do prove effective have long-lasting benefits and there is hope that use of a combination of therapies will improve response rates.
Figure 2 uses graphs to demonstrate the long-term efficacy of using ICIs to treat advanced cancer. Because ICIs are less prone to the drug resistance that afflicts chemotherapy using drugs that target specific molecules, they have been shown to deliver significant long-term benefits for certain patients(1), (5). Unfortunately, even with the use of biomarkers, the response rate (proportion of patients who show such benefits) is low, no more than 40 to 50%(6), making it important to develop biomarkers that can identify what is blocking immune functions and indicate which combination therapy will work best.
Companion diagnostics is the term used for tests conducted prior to administration of a drug to determine its efficacy and side effects. As of September 2020, the following four companion diagnostic tests for ICIs were approved for use in the USA(1), (2).
While the first two of these tests are most commonly used, a statistical meta-analysis of multiple clinical trials has found that they are not sufficiently accurate(6). For example, the positive predictive value (PPV) (the proportion of true positive results) for the PD-L1 and TMB tests are 0.40 (0.36 to 0.45)* and 0.54 (0.47 to 0.60)* respectively, meaning that about half of all patients identified as candidates for the treatments do not in fact benefit from them. The MSI and dMMR tests likewise do not perform well, with PPVs of only 0.50 and 0.53 respectively when applied to colorectal cancer patients(7), (8). All of these tests target tumor cells. While a wide variety of tests intended to assess the state of a patient’s immune system have been developed in Japan and elsewhere, none of these are yet able to offer high accuracy(1). Particular promise is seen in the discovery of accurate biomarkers in testing for the activity of tumor-infiltrating lymphocytes. Testing that combines a number of different biomarkers and analyses has been proposed as a way to improve diagnostic accuracy(1). An important factor when combining different tests is that they be independent of one another. Although PD-L1 and TMB testing meet this criterion, trials of their use in combination have failed to deliver adequate accuracy(6). This has directed attention toward clinical research aimed at improving accuracy by using these existing biomarkers in tandem with other biomarkers associated with immune response. Along with the use of flow cytometry to classify cells, single-cell analyses that sort individual immune cells by function have also proved effective(9).
Prompted by the availability of NGSs, success has been achieved with precision medicine that makes use of the patient’s genetic information (DNA sequences)(3). Meanwhile, the increasing number of candidate ICI therapies and the development of various combination therapies offer new possibilities for precision medicine. Specifically, comprehensive clinical data on genetic abnormalities in tumor cells has been used with TMB testing as a companion diagnostic technique for ICIs(1). By applying this method to a wide range of immune cells to acquire evidence about the cancer immune response, it is anticipated that it can be used in the development of precise companion diagnostic technique for ICIs. As impediments to the cancer-immunity cycle differ from patient to patient, ongoing testing for disease progress has potential as a form of personalized medicine, helping identify therapies with a high probability of success based on the actual state of the patient’s immune system.
Since 2012, Hitachi has been developing vertical flow array chips (VFACs), devices for single-cell gene analysis that rely on the acquisition of data by NGSs. The company is now seeking to establish methods for highly accurate immune cell testing that make practical sense in the context of the state of progress discussed above(10).
As many different cell types are involved in the functioning of immune cells, the ability of flow cytometry to perform cell-by-cell analyses makes it a standard technique in immunology. Meanwhile, the falling cost and wider availability of NGSs over recent years offer a potential solution to the quantitative failings of flow cytometry(1), (11). Specifically, DNA barcoding is recognized as a way to improve the accuracy with which gene expression can be quantified(12), (13).
NGS analysis using DNA barcodes is a common technique for the bulk sequencing of large numbers of samples. DNA barcodes play two important roles in single-cell analysis. The first is to identify particular cells in sequence data from a mixture of many different cells. The second is to use the NGS to identify the genes expressed in individual cells at the molecular level by means of messenger ribonucleic acid (mRNA). Moreover, single-cell analysis using DNA barcodes is able to provide absolute counts for the number of cells and molecules present. This is in contrast with conventional analysis methods that do not use DNA barcodes and are only able to provide relative quantifications of gene expression because sequencing only covers a subset of the input sample.
Figure 3 — Schematic Diagram of VFACs
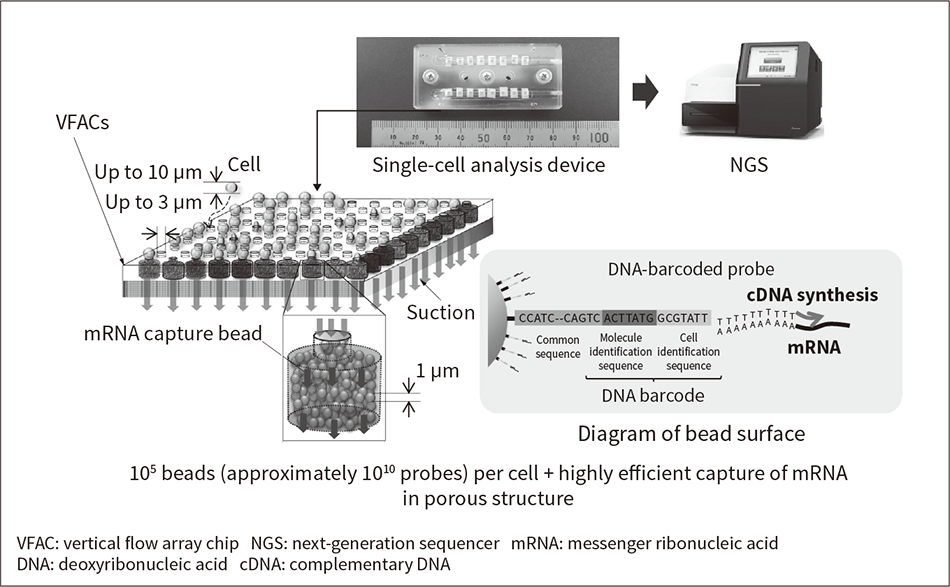 In addition to cutting costs by introducing DNA barcodes into a large number of cells simultaneously to prepare a bulk sample for the NGS, the barcoding of mRNA can also be performed efficiently by matching each cell with large numbers of beads coated with DNA-barcoded probes.
In addition to cutting costs by introducing DNA barcodes into a large number of cells simultaneously to prepare a bulk sample for the NGS, the barcoding of mRNA can also be performed efficiently by matching each cell with large numbers of beads coated with DNA-barcoded probes.
Hitachi has developed VFAC devices for single-cell analysis, as shown in Figure 3(10). As well as providing sensitive and accurate quantitative gene analysis by the efficient DNA barcoding of mRNA derived from individual cells, savings can also be made in the cost of reagents by processing large numbers of cells together in the same VFAC. The cell capture pores in the VFAC shown in Figure 3 are filled with beads coated with 1010 molecules of mRNA-capture DNA probes (which include DNA barcodes), enabling them to efficiently capture mRNA molecules (105-6) from individual cells. The sequence data is obtained by a series of steps performed after capture and from this the mRNA molecules in the cells can be recovered more efficiently than by other methods(10).
Figure 4 — Platform for Cancer Immunotherapy Development and Clinical Testing for Therapy Selection
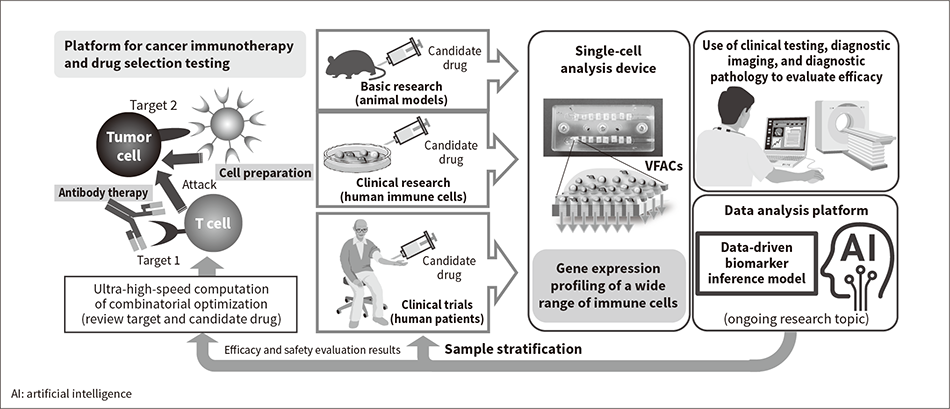 By enabling large numbers of cell analyses with low running costs as well as sensitive and accurate quantitative gene analysis, VFACs are suitable for everything from basic research under a wide range of conditions to clinical trials that demand accuracy and involve large numbers of samples.
By enabling large numbers of cell analyses with low running costs as well as sensitive and accurate quantitative gene analysis, VFACs are suitable for everything from basic research under a wide range of conditions to clinical trials that demand accuracy and involve large numbers of samples.
Biomarkers for cancer immunity can be used by means of machine learning both in drug development (especially the development of combination therapies) and in clinical testing for therapy selection (personalized medicine). As shown in Figure 4, the VFACs currently under development allow for different quantities and sizes (number of capturable cells) of VFACs to be used on devices to enable the same device platform to be used both in the evaluation of biomarkers for drug development and in biomarker-based clinical testing.
As part of joint research with the Kakimi Laboratory, Department of Immunotherapeutics, The University of Tokyo Hospital, VFACs were used for single-cell analysis to study the interaction between immune cells and the micro-environment in mouse stomach cancer and to assess the antitumor effect of therapeutic agents(14).
The research involved a single-cell analysis of T cells that had infiltrated stomach cancer tissue in mice with the objective of measuring the expression of 44 immune-related genes and then assessing whether it is possible to identify the key signals involved in the cancer immune response.
Two stomach cancer tumor cell lines (YTN2 and YTN16) were introduced into healthy mice (C57BL/6N) to prepare two separate models of stomach cancer (YTN2 mice and YTN16 mice). Whereas the YTN2 mice experienced low malignancy with immune functions eliminating the tumors after about 20 days, the YTN16 mice had high malignancy with tumor size increasing over time. T cells obtained by a cell-sorter from the tumor-infiltrating lymphocytes (on day 7 after transplantation of the tumor cell lines) were dispensed on VFACs and the prepared samples analyzed by an NGS.
The analysis provided expression profiles for 44 immune-related genes from 1,098 T cells in total (from both the YTN2 and YTN16 mice).
Figure 5 (a) shows a two-dimensional scatter plot that has been color-coded by Louvain clustering (without learning) and Figure 5 (b) shows a heat map. Cluster 6 is made up exclusively of cells from YTN16 mice and is notable for the high level of expression for the IL-17 gene, as highlighted by the green arrow. This suggests that the IL-17 gene plays a role in cancer immunosuppression in YTN16 mice.
To confirm the function of the highlighted IL-17 gene, tumor sizes were compared for a control group that received no treatment, a group that received only anti-IL-17 antibodies, another that received only anti-PD-1 antibodies (an ICI), and a group that received both [see Figure 5 (c)]. This found a strong antitumor effect with rapid elimination of the tumor in eight out of ten mice in the dual-treatment groups. This result indicates that single-cell analysis using VFACs can identify genetic alteration signals that relate to cancer immunity. It also demonstrated the strong antitumor effect of immunotherapy based on the identified signals. A feature of expression associated with IL-17 is that it cannot be detected by conventional RNA sequencing, which demonstrates another benefit of single-cell analysis(14).
Figure 5 — Results of Using VFACs for Single-cell Analysis of T Cells in Mouse Tumor and Assessment of Inhibitory Effect on Cancer of Identified Signals
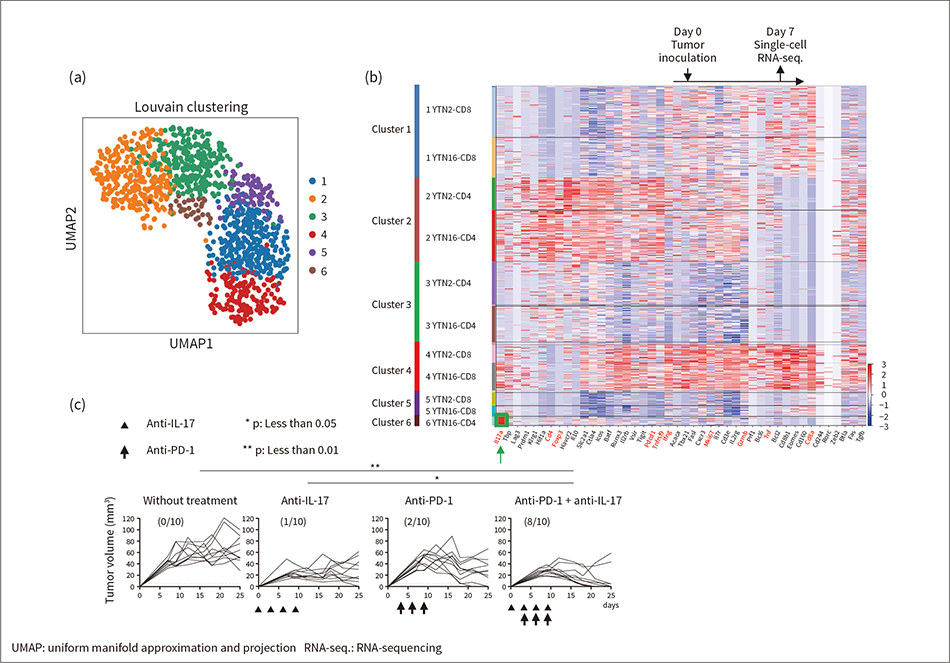 Graph (a) is a two-dimensional projection (using the UMAP technique for dimension reduction) of the Louvain clustering (without learning) of single-cell results obtained by VFACs. Graph (b) shows the same results as a heat map. The graphs in (c) show the reactivation of cancer immunity that has been inhibited by one of the identified signals (IL-17).
Graph (a) is a two-dimensional projection (using the UMAP technique for dimension reduction) of the Louvain clustering (without learning) of single-cell results obtained by VFACs. Graph (b) shows the same results as a heat map. The graphs in (c) show the reactivation of cancer immunity that has been inhibited by one of the identified signals (IL-17).
Immunotherapy for cancer has attracted attention in recent years, with rapid advances being made in development and clinical trials of optimal treatment schemes that include combination therapies. While the research and development of biomarkers for identifying the best therapies is an increasingly active field, it remains a work in progress. Hitachi will continue to contribute to the treatment of cancer by combining digital technology with immunological testing for cancer to reduce costs while also striving to put these to use for a wide variety of other conditions where immune response is relevant, such as infectious disease.
The evaluation of the VFAC devices for performing single-cell analyses described in this article benefitted from the assistance of numerous academics, including Professor Kazuhiro Kakimi of The University of Tokyo Hospital. The authors would like to express their deep gratitude for this help.