Preventing the microbial contamination of pharmaceuticals requires the control of microorganisms in both products and the production environment. The problem with conventional methods, unfortunately, is that they can take several days or more to complete. In particular, the cell-based preparations used in regenerative medicine will likely generate greater demand than ever for rapid microbial testing because of their short shelf lives and because they contain living cells and so do not allow for sterilization. By developing the rapid microbial testing system Lumione BL-2000 that can detect microorganisms with high sensitivity by means of ATP testing, Hitachi has succeeded in increasing the speed of microbial testing for pharmaceutical-grade water and high- and low-molecular-weight pharmaceuticals. This article describes the development of technologies for faster microbial testing of production environments and cell-based preparations that are currently being undertaken to expand use of ATP testing.




To ensure patient safety, pharmaceutical manufacturing is obliged to guarantee certain levels of quality by preventing product contamination across all steps, from raw materials through to product production, processing, and dispatch. As microorganisms are one of the main sources of contamination risk in pharmaceuticals, dealing with them is a key business requirement. To achieve this, manufacturers conduct microbial testing across the entire production process, encompassing raw materials, intermediate products, end products, pharmaceutical-grade water, and the production environment.
As the bacteria and fungi (microorganisms) being tested for are in the 1- to 10-µm range, detecting a small number of these in a sample by means of an optical microscope is not practical. The conventional method was to use culturing, whereby samples were cultured on a growth medium to increase their number sufficient to enable visual detection. For a microbial enumeration test, the sample would be cultured on agar for several days. Sterility testing using a liquid culture medium, on the other hand, must be done for 14 days(1), (2). Replacing these methods with rapid microbial testing has the potential to enhance pharmaceutical quality management by enabling the rapid detection of microorganisms while also reducing manufacturing costs and speeding up production and dispatch by shortening the waiting time for obtaining test results. In particular, there is an urgent need for rapid microbial testing of end products in the field of regenerative medicine that has seen rapid progress over recent years. This is because the cell-based preparations used in regenerative medicine often have a shelf life in the order of a few days and are unable to use practices such as heat sterilization because they contain living human cells(3). Regulatory considerations also suggest that rapid microbial testing will likely become more widely used in the future, with the practice being listed in the 17th edition of the Japanese Pharmacopoeia published in 2016 and consideration of its use being recommended in the 2019 guidance for the aseptic manufacturing process of regenerative medicines issued by the Ministry of Health, Labour and Welfare of Japan(4).
Hitachi developed the rapid microbial testing system Lumione BL-2000 (hereinafter “Lumione”) to improve control of microorganisms in pharmaceutical manufacturing and thereby help to enhance the quality of life (QoL) of patients. To achieve faster microbial testing, the system uses a highly sensitive technique for the detection of microorganisms called adenosine triphosphate (ATP) testing that works on the principle of bioluminescence(5).
As all living cells use ATP as a means of supplying energy to the metabolic process, it is also found in microorganisms. ATP testing uses photometry to quantitatively measure the amount of ATP with the help of the luciferin-luciferase reaction for highly efficient light generation that was discovered to be the principle behind luminescence in fireflies. In the past, the lower detection limit for ATP testing was around 100 amol (1 amol = 10−18 mol).
For Lumione, Hitachi achieved high sensitivity with a lower ATP detection limit of 0.3 amol by developing special reagents (pretreatment reagent and luminescence reagent) and a luminometer able to detect very low levels of luminescence. As many microbial strains have an ATP content, defined as the amount of ATP per colony-forming unit (CFU) obtained from the plate-count method, of 1 amol or higher, this ability of Lumione to detect ATP at levels equivalent to a single CFU of microbes is a ground-breaking advance.
Figure 1 shows how measurement is performed by Lumione. For pretreatment, the sample is passed through a filter with a pore size of 0.45 µm to concentrate the microorganisms, with the liquid filtrate being discarded. A reaction is then used to eliminate the extracellular ATP in the material collected on the filter and the intracellular ATP is extracted using a small quantity of reagent. This increases microbial detection sensitivity by obtaining a sample with a high-concentration of microbial ATP. This reagent has been modified to also enable the extraction of ATP from bacterial spores. Measurement tubes containing the resulting ATP samples are inserted into Lumione along with the luminescence reagent and the analysis is started. Inside Lumione, the measurement tubes are automatically transferred to the luminometer one at a time where luminescence reagent is added and measurement is performed. Up to 24 measurement tubes can be inserted at a time. As measurement takes 5 minutes for each tube, 2 hours is required to analyze all 24 tubes.
In a previously reported application of the Lumione in pharmaceutical manufacturing involving the analysis of samples from pharmaceutical-grade water and high- and low-molecular-weight pharmaceuticals, it was able to detect 1 CFU of test strains in just one hour, including pretreatment(5). This performance has the potential to reduce manufacturing costs by improving plant utilization through shorter startup times for pharmaceutical-grade water equipment and by speeding up the dispatch of pharmaceutical products. Hitachi is also developing testing protocols that will better meet diverse requirements for faster microbial testing.
The following sections describe techniques currently under development for speeding up environmental testing and the sterility testing of cell-based preparations.
Figure 1 — Lumione Measurement Procedure To improve sensitivity, the microorganisms in the sample are first collected in a filter and then undergo a pretreatment process that takes about one hour. Lumione automatically dispenses luminescence reagent into each measurement tube and performs a luminosity measurement. Up to 24 measurement tubes can be inserted at a time. Measurement takes 5 minutes for each tube, or 2 hours for all 24 tubes.
To improve sensitivity, the microorganisms in the sample are first collected in a filter and then undergo a pretreatment process that takes about one hour. Lumione automatically dispenses luminescence reagent into each measurement tube and performs a luminosity measurement. Up to 24 measurement tubes can be inserted at a time. Measurement takes 5 minutes for each tube, or 2 hours for all 24 tubes.
Figure 2 — Sterility Testing Using Surface Swabs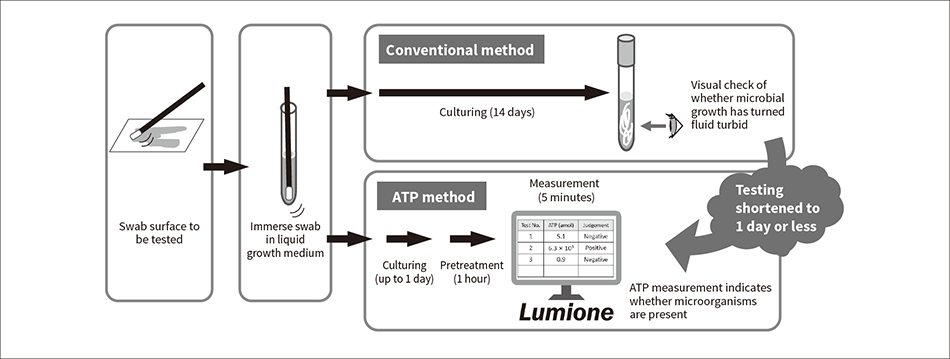 Whereas the conventional visual testing method required 14 days of culturing, the ability of ATP testing to detect microbes with high sensitivity has shortened the testing time to between a few hours and one day.
Whereas the conventional visual testing method required 14 days of culturing, the ability of ATP testing to detect microbes with high sensitivity has shortened the testing time to between a few hours and one day.
Microbial management of the production environment for pharmaceuticals involves passive and active monitoring for airborne microorganisms and surface monitoring. In particular, it includes managing the sterilization of the aseptic workplaces used to manufacture injectables and other sterile preparations.
Figure 2 shows how such testing can be performed using surface swabs to verify surface sterility. The conventional method was to use commercially available swabs (cotton buds) for sample collection on the surfaces of interest, with these swabs then being soaked in a liquid growth medium for culturing. After 14 days the sterility of the surface was assessed by visually checking whether microbial growth had turned the fluid turbid. ATP testing, in contrast, performs pretreatment and measurement on Lumione after only a short incubation time. If the resulting ATP measurement is below a predefined threshold, the sample can be judged to be sterile. Whereas visual identification of microorganisms requires that they must have multiplied in number to around 107 CFU, the ability of ATP testing to detect them at much lower numbers means that it can be carried out after culturing for a much shorter time. The length of this incubation time is determined by the extent to which the ATP in the sample itself obstructs measurement, the microbial ATP content, and growth rate.
Table 1 lists the results of a study that looked at the use of ATP sterility testing to check for microbial surface contamination on staff involved in aseptic manipulation. The samples were commercially available cleanroom products (sterile nitrile gloves and sterile polypropylene sleeve protectors). A measurement to determine how much ATP is present on the materials from which these products are made, was taken from swabs of 10 cm × 10 cm surfaces (using off-the-shelf swabs). The result was less than 1 amol, indicating that a threshold of 1 amol could be set for microbial detection. Next, swabs were contaminated with 15 CFU of Cutibacterium acnes (C. acnes, DSM 1897), a slow-growing bacterium that could easily be carried in by staff, and 21 CFU of Pseudomonas aeruginosa (P. aeruginosa, ATCC 9027), the bacterial strain with the lowest ATP content among those specified in the Japanese Pharmacopoeia as able to be used in the validation of sterility testing procedures. These were incubated for 4 hours and then analyzed for ATP. The results demonstrated that ATP testing can successfully be used for microbial detection.
Future plans include swabbing surfaces in actual production environments and building up data on microbes present in the environment in order to establish testing protocols with a view to the commercialization of same-day sterility testing.
Table 1 — Example Measurement of Surface Swabs The results demonstrate that the amounts of ATP present on swabs taken from commercially available sterile cleanroom gloves and sleeve protectors are less than 1 amol, too low to interfere with microbial detection. The measured values for C. acnes and P. aeruginosa also indicate that, when starting from an initial microorganism count of 1 CFU, the amount of microbial ATP present after 4 hours of incubation is several amol, enough to be detected by ATP testing.
The results demonstrate that the amounts of ATP present on swabs taken from commercially available sterile cleanroom gloves and sleeve protectors are less than 1 amol, too low to interfere with microbial detection. The measured values for C. acnes and P. aeruginosa also indicate that, when starting from an initial microorganism count of 1 CFU, the amount of microbial ATP present after 4 hours of incubation is several amol, enough to be detected by ATP testing.
Figure 3 — Sterility Testing Procedure for Cell Sample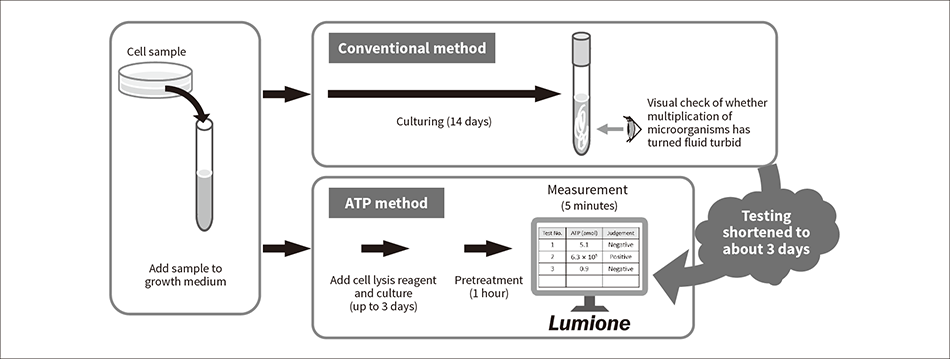 In order to detect a small number of microorganisms present amid a large number of human cells, the technique combines culturing with use of a newly developed cell lysis reagent reagent to remove human cells. By doing so, the sterility testing time (using ATP testing) is reduced to just 3 days.
In order to detect a small number of microorganisms present amid a large number of human cells, the technique combines culturing with use of a newly developed cell lysis reagent reagent to remove human cells. By doing so, the sterility testing time (using ATP testing) is reduced to just 3 days.
Figure 4 — Example Measurement for Detecting Microbes in Cell Samples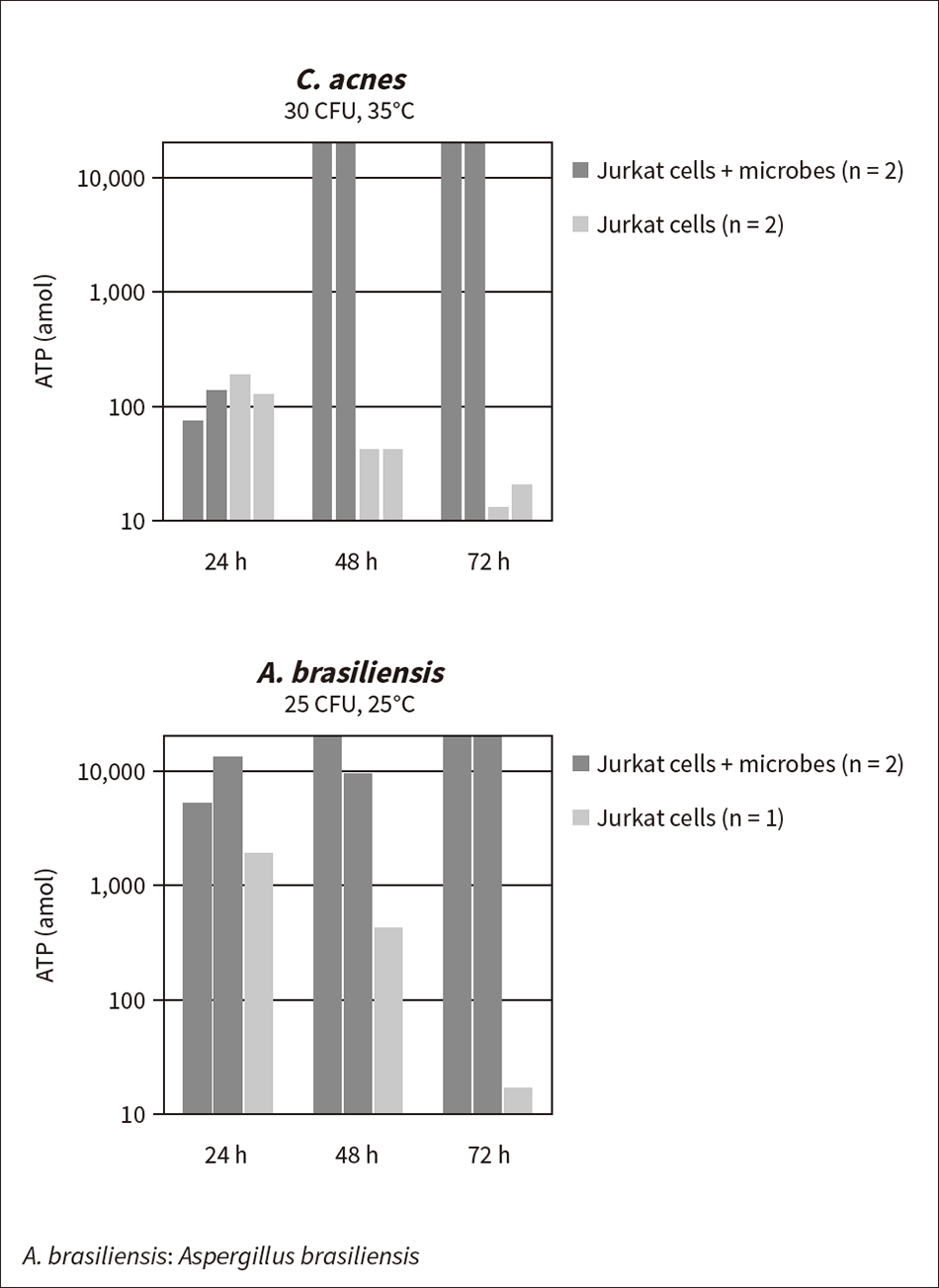 The addition of a cell lysis reagent causes the ATP from the Jurkat cells to diminish over time. Meanwhile, microbial growth causes an increase in ATP. The quantity of ATP after 48 hours of culturing was 10 or more times higher in samples containing cells to which C. acnes or A. brasiliensis had been added than in equivalent sterile samples, indicating that microbe detection is possible.
The addition of a cell lysis reagent causes the ATP from the Jurkat cells to diminish over time. Meanwhile, microbial growth causes an increase in ATP. The quantity of ATP after 48 hours of culturing was 10 or more times higher in samples containing cells to which C. acnes or A. brasiliensis had been added than in equivalent sterile samples, indicating that microbe detection is possible.
Cell-based preparations by their nature are unable to undergo sterilization because they contain living human cells. Instead, every step of the manufacturing process takes place under aseptic conditions and a sterility test is performed on the end product. Figure 3 shows one example of the sterility test procedure.
Samples that contain human cells are a problem because they interfere with the test. With the conventional method, the cells present in the sample would themselves cause the microbial culture medium to become turbid, thereby hindering the visual check for microbial growth. Likewise with ATP testing, the ATP from the cells interferes with the detection of microorganisms. If a sample contains around 107 human cells, for example, the amount of ATP they contain is roughly equivalent to that of 108 bacteria. In other words, it would still not be possible to detect the bacteria even if culturing multiplied their number to 108. Moreover, the cells cannot be removed by filtering because they are similar or larger in size than the microorganisms.
To overcome this problem and enable ATP testing to be used for the rapid detection of microbes, Hitachi developed a cell lysis reagent that does not interfere with microbial growth but works selectively on human cells so that they can be removed by filtering. The required culturing time depends on the microbe’s ATP content and growth rate as well as on the microbe detection threshold, which in turn is determined by the amount of residual human cell ATP.
Figure 4 shows an example of microbe detection in a cell sample using the cell lysis reagent. A sample containing Jurkat cells (a line of human T lymphocyte cells) was prepared to replicate the sort of human T-cell preparations that are used in immune cell therapy or gene-modified cell therapy. A 1-mL sample was prepared containing 107 cells/mL of Jurkat cells in suspension (solvent: Ringer’s solution with 5% fetal bovine serum, 10% dimethylsulfoxide). As the model microbial strain, 30 CFU of C. acnes or 25 CFU of Aspergillus brasiliensis (A. brasiliensis, ATCC 16404), a fungus that is one of the microorganisms used for the validation of sterility testing procedures was added. In accordance with the Japanese Pharmacopoeia, the C. acnes were cultured at 35°C and the A. brasiliensis at 25°C, both in the presence of the cell lysis reagent. At the start of the test, the quantity of ATP from the Jurkat cells was approximately 107 amol. Through the action of the cell lysis reagent, this quantity reduced by six orders of magnitude over a period of 72 hours, to about 10 amol. While the reduction rate is higher at higher temperatures, for both strains of microorganism, the quantity of ATP after 48 hours of culturing was 10 or more times higher in cell samples to which microorganisms had been added than in equivalent sterile samples, which enabled the detection of microorganisms. Allowing for variability in the condition of the cells and in microbial growth, this indicates that an incubation time of around 72 hours is sufficient for verifying sterility.
Meanwhile, Hitachi is currently developing a cell lysis reagent that is even more effective at cell removal with the aim of producing a rapid sterility test that can obtain same-day results from a cell sample. This includes building up data on other strains of cells and microbes in preparation for practical deployment.
Through the development of the rapid microbial testing system Lumione for the detection of microorganisms with high sensitivity, Hitachi is contributing to product quality management and faster microbial testing in pharmaceutical manufacturing. Along with the testing of pharmaceutical-grade water and sterility testing of high- and low-molecular-weight pharmaceuticals, this has also included looking at using the system for environmental testing and for sterility testing of cell-based preparations.
In the future, Hitachi will continue developing technology for faster microbial testing of raw materials and for in-process testing, also using collaborative creation with customers to enhance and deploy testing practices that meet the needs of the manufacturing workplace. Hitachi also intends to contribute to improvements in microbial management at a wide variety of manufacturing plants by extending use of these new technologies to other industries such as cosmetics and food.