We have a history of over 60 years supplying fermentors and fermentation plants. Please inquire with us about construction of pharmaceutical plants using microbial fermentation or cell culture. We can help improve productivity through optimization of fermentors by actual fluid culture testing and simulations. We also support our customers' initiatives to strengthen GMP compliance. We support our customers with reliable technology and the strongest delivery track record in Japan.

There are two main types of processes used to manufacture pharmaceuticals: chemical synthesis, based on chemical reactions, and bioprocessing based on the ability of microorganisms and cells to produce useful substances. Antibiotics are one representative category of pharmaceutical product produced using the bioprocessing. In recent years, the spotlight has been on antibiotics produced using animal cells.
New methods for producing influenza vaccine can produce large quantities of vaccine more efficiently, compared with conventional methods using poultry eggs. Currently, the bioprocessing is the only production method in use for making drugs as molecularly complex as proteins or viruses, with low levels of side effects. We are working to develop the most up-to-date culture technology, to meet our customers' needs in all areas of bioprocessing pharmaceutical production.
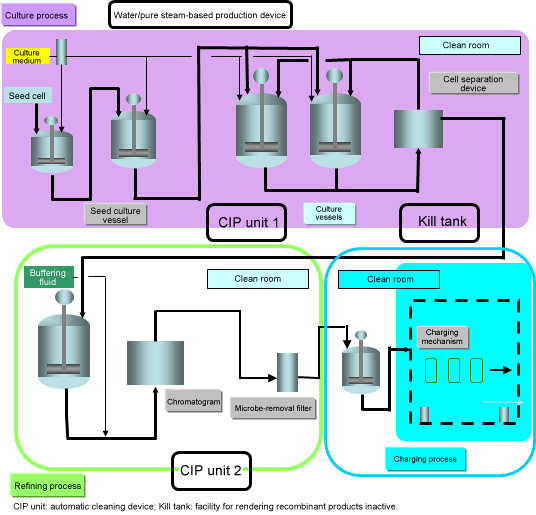
Pharmaceuticals manufacturing process using cell culture
We can provide an EPC package combining engineering, procurement, construction, and validation are possible.
Contact us from here.