Featured customer case
Lumada customer case code: UC-01900S
The Hitachi Value Chain Traceability service for Regenerative Medicine (HVCT RM) facilitates connection of processes in regenerative medicine and enables successful completion of a cell’s journey
2023-06-29

Regenerative medicine*1 is drawing attention as a new form of medicine that utilizes cells from a patient’s own body. In order to successfully complete a cell’s journey, which is a series of connected safe and reliable processes from collecting the cells to administering the resulting product (the therapeutic agent) into patients, medical service providers must centralize management of information about a regenerative therapy, establish traceability, and connect the series of all these processes. This article provides a customer case in which a medical service provider manages information and traceability in the entire value chain to maintain and improve the safety of regenerative medicine.
Many stakeholders are involved in providing regenerative-medicine products*2, including medical institutions, pharmaceutical companies, logistics companies, and manufacturing companies. If all these stakeholders use the same cloud service platform, they no longer need to separately use and maintain their own systems to share information such as patient information, planned schedules, quality information, or transportation results. Such a cloud service platform enables centralized management of information owned by all the stakeholders and thereby enables application of specialty drugs to rare diseases. Global deployment of such a platform will contribute to improving corporate value and lifting the quality of life (QoL) of many people.
Co-creation with Lumada―Managing information throughout the entire value chain and ensuring traceability
Expectations for regenerative medicine are growing as it provides therapies that are the last resort for curing rare or intractable diseases for which there have been no effective remedies.
Regenerative medicine can restore a tissue, an organ, and the health of people who lost those functions as a result of a disease or injury. Matters related to safety and quality management of regenerative-medicine products are prescribed by laws*3 to ensure that new therapies in this field will be delivered to as many patients as possible.
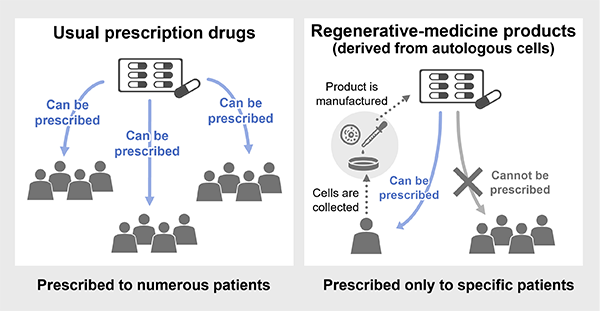
Regenerative medicine uses products tailored to meet the specific needs of one patient or a small number of patients. Although it is essential that regenerative therapies maintain the quality of conventional pharmaceutical drugs, it is also essential that information management be implemented in a way that clearly links the patient, cells, and the product manufactured from the cells.
Currently, however, stakeholders (such as medical institutions, logistics companies, pharmaceutical companies, and manufacturing companies) are sharing information by telephone, email, paper, or other means. Communication by telephone or email requires time and effort, and information recorded on paper is subject to the risk of inaccuracy, tampering, and leakage. There is a strong demand to resolve this undesirable situation in order to ensure safety and quality.
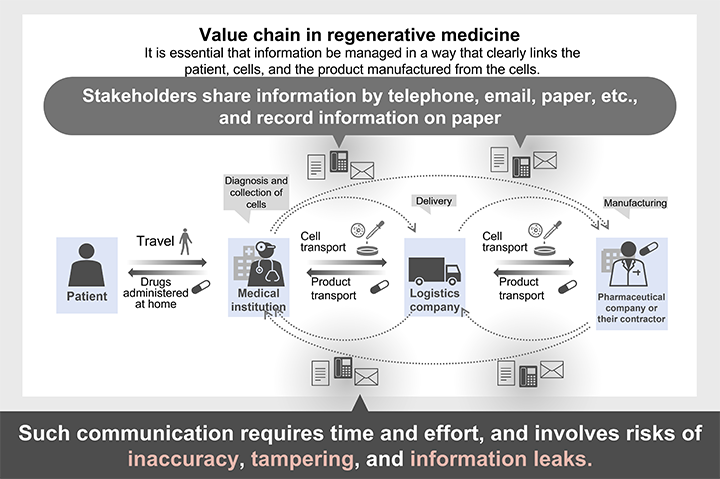
If all the stakeholders involved can share information about who does what and when, they will be able to communicate smoothly and closely and maintain and improve the safety of regenerative medicine.
Hitachi provides a service platform that can be used by all stakeholders, enabling centralized management of information throughout the entire value chain. Stakeholders can save time and effort when sharing information about work progress and coordinating schedules and ensure safety and quality.
Hitachi’s cloud services can be combined to properly manage the permissions users need to access centrally managed information. These services can be used to manage records of business activities and verify samples in compliance with pharmaceutical regulations and personal information protection requirements.
Managing information without depending on a specific type of device
To enter and view information, users can use browsers on a variety of devices such as PCs, tablets, and smartphones. Data is easy to handle because it is linked with the customer’s system.

Making operations easier
By using a browser to perform the operations required for the processes in regenerative medicine, users can perform operations in workflow-like procedures.

Ensuring a high level of safety by setting access permissions
Administrators can set user permissions to restrict which features are available to a user. In addition, the system automatically anonymizes and encrypts data before saving and storing it, thereby achieving a high level of safety.

Data visualization
Users can check the business relationships among stakeholders and the work progress on windows displaying the value chain. The user can quickly identify the progress because data is visually presented.
These features enable visualization of the entire value chain and provide the following benefits.
Our solution adopts IoT Compass, a cloud service from Hitachi, to centralize management and provide visualization of massive amounts of data. In the customer case featured below, IoT Compass linked with data in the factory, thereby improving traceability and improving safety and efficiency in production activities.
The next page introduces a solution that utilizes centrally managed data in order to facilitate smooth information sharing among stakeholders and to achieve high levels of safety.