Support for traceability management and improvements in work efficiency, based on enhanced cooperation
Hitachi Value Chain Traceability service for Regenerative Medicine―An integrated value chain management platform for regenerative-medicine products
Hitachi Value Chain Traceability service for Regenerative Medicine (hereinafter abbreviated as HVCT RM) is a SaaS service available for all stakeholders involved in a value chain. HVCT RM enables various stakeholders to smoothly share information and ensures a high level of safety.
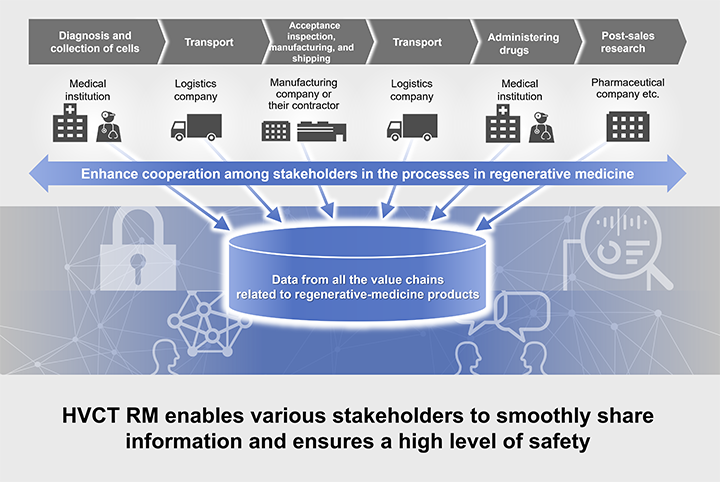
The IT platform supports industry standard processes for enhanced cooperation among stakeholders
HVCT RM leverages an IT platform that can cover the entire value chain and ensures safety and efficiency improvements in the processes in regenerative medicine, which involve many different stakeholders. HVCT RM has the following three special features.
- (1) Enables smooth and safe cooperation among stakeholders
- Implements centralized data management to enable smooth information sharing throughout the entire value chain
- Manages permissions to ensure a high level of security and safe cooperation
By leveraging this feature, you can streamline and reduce communication and coordination tasks. The feature also helps you prepare periodic reports and other documents.
- (2) Ensures traceability
- Manages the traceability of processes ranging from collecting samples to administering therapeutic agents.
- Manages evidence of operations throughout the entire value chain.
This feature helps to reduce the risk of sample mix-ups and ensures safety. If an issue occurs, users can promptly trace the issue and quickly identify its causes.
- (3) Automatically generates and shares therapy schedules
- Checks resource availability and automatically generates schedules
- Quickly responds to changes in a schedule
This feature helps to reduce the burden on stakeholders who need to coordinate the work. Up-to-date information is shared continuously, making it possible to ensure transparency for stakeholders and patients. Data visualization of the status of operations simplifies the task of formulating maintenance schedules for facilities and equipment.
Three features of HVCT RM
HVCT RM provides the following three functionalities to ensure enhanced cooperation among stakeholders:
- Functionality for collecting data
-
- By use of a value chain framework configurable for each customer and a window for registering task results, users can use a browser to specify customer operations. Users can thereby perform operations in a workflow format.
- Users can view and enter information on a browser on a variety of devices such as PCs, tablets, and smartphones. The data can be handled easily because it is linked with customer systems.
- Functionality for managing patient information
-
- Personal information about patients can be anonymized and encrypted for safe management.
- Functionality for controlling data access permissions
-
- Intuitive displays of the connections between business processes and the progress of business processes are shown on the value chain framework, so users can visually check this information.
- By assigning permissions to users, administrators can control data access, data input, and features available to users.
Example of applying the solution
We will now describe a case example of Alfresa Corporation*4, which introduced HVCT RM and thereby achieved smooth information sharing and efficient business operations.
It is difficult for companies in a complex value chain to jointly establish processes, business rules, and means of communication from scratch in a coordinated manner. This is one of the obstacles in advancing regenerative medicine. By introducing HVCT RM, Alfresa gained the following benefits.
- They could establish business processes at an early stage, because HVCT RM had already defined the series of processes in a value chain and provided a standard model of necessary records.
- They could reduce the risk of medical malpractice and could quickly identify the causes of problems, because HVCT RM enabled users to trace task results.
- They achieved robust security of patients’ personal information without effort, because such information was automatically anonymized and encrypted before it was saved and stored.
- They decreased patient waiting times as a result of smooth information sharing among stakeholders and smooth coordination of schedules. This resulted in an increase in therapeutic opportunities, and enabled smoother provision of therapies.
- *4
- News release (Aug. 31, 2020)
Outlook for the future
HVCT RM is expected to further increase value, as explained below.
- We plan to add a data analysis feature to expand the scope of optimization of processes in regenerative medicine. For example, HVCT RM will be able to detect irregular tasks occurring in the value chain. In addition, users will be able to perform simulations using different statuses of various resources.
- In the future, HVCT RM is likely to support functionality for monitoring the conditions of patients to which regenerative-medicine products are administered. When this functionality is supported, HVCT RM users will be able to analyze the patient conditions against detailed requirements (such as those related to manufacturing and delivery) and thereby find the best manufacturing or rehabilitation method that will have the optimum therapeutic effect on patients.
- Initially, HVCT RM was intended to be used for products derived from autologous cells. Since then, HVCT RM has increasingly been used for products derived from allogeneic cells or products based on laws related to ensuring the safety of, for example, regenerative-medicine products. In the future, HVCT RM is expected to become applicable to specialty drugs*5 and other types of products*6.
When these planned features are supported, HVCT RM will contribute more to the development of regenerative medicine.
- *5
- Specialty drugs include expensive drugs, biological drugs, and regenerative-medicine products (for example, drugs for rare diseases that require strict temperature management, inventory management, and security management).
- *6
- Regenerative-medicine products (Hitachi Pharma Information Solutions) (Japanese)
For details on our solutions, see the following webpages.
- Integrated value chain management platform for regenerative-medicine products
- Hitachi Value Chain Traceability service for Regenerative Medicine
-
This solution can be provided to customers in any region.
- A shared service platform for stakeholders related to regenerative-medicine products
- Hitachi Value Chain Traceability service for Regenerative Medicine―An integrated value chain management platform for regenerative-medicine products
-
This solution can be provided to customers in any region.
For more details on the related technologies, please read the following articles:
- Patient registry service
- A highly secure cloud registry enables users to efficiently collect and use high quality information about patients.
- "HITPHAMS" pharmaceutical manufacturing execution system
- The information network reaches all corners of the manufacturing site. It therefore creates an advanced production environment.
